Toluidine
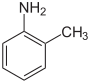
There are three isomers of toluidine, which are organic compounds. These isomers are o-toluidine, m-toluidine, and p-toluidine. The o- stands for ortho-, m- stands for meta- , and p- stands for para- . All three are aryl amines whose chemical structures are similar to aniline except that a methyl group is substituted onto the benzene ring. The difference between these three isomers is the position where the methyl group (-CH3) is bonded to the ring relative to the amino functional group (-NH2); see illustration of the chemical structures below.
| Toluidine isomers | |||
|---|---|---|---|
| Common name | o-toluidine | m-toluidine | p-toluidine |
| Other names | o-methylaniline 2-methylaniline |
m-methylaniline 3-methylaniline |
p-methylaniline 4-methylaniline |
| Chemical name | 2-amino-1-methylbenzene | 3-amino-1-methylbenzene | 4-amino-1-methylbenzene |
| Chemical formula | C7H9N | ||
| Molecular mass | 107.17 g/mol | ||
| Glass transition temperature | 189 K[1] | 187 K[2] | [no glass former][1] |
| Melting point | −23 °C | −30 °C | 43 °C |
| Boiling point | 199–200 °C | 203–204 °C | 200 °C |
| Density | 1.00 g/cm3 | 0.98 g/cm3 | 1.05 g/cm3 |
| CAS number | [95-53-4] | [108-44-1] | [106-49-0] |
| SMILES | Cc1ccccc1N | Cc1cccc(N)c1 | Cc1ccc(N)cc1 |
 |
 |
| |
| Disclaimer and references | |||
The chemical properties of the toluidines are quite similar to those of aniline and toluidines have properties in common with other aromatic amines. Due to the amino group bonded to the aromatic ring, the toluidines are weakly basic. None of the toluidines is very soluble in pure water, but will become soluble if the aqueous solution is acidic due to formation of ammonium salts, as usual for organic amines. At room temperature and pressure, ortho- and meta-toluidines are viscous liquids, but para-toluidine is a flaky solid. This can be explained by the fact that the p-toluidine molecules are more symmetrical and fit into a crystalline structure more easily. p-Toluidine can be obtained from reduction of p-nitrotoluene. p-Toluidine reacts with formaldehyde to form Tröger's base.
Toluidines are used in the production of dyes. They are a component of accelerators for cyanoacrylate glues. They are toxic and are suspected human carcinogens.
The related compound o-tolidine, used as a presumptive test for blood in forensic science, is two o-toluidine molecules linked together.
References
- ↑ 1.0 1.1 Pratesi, G.; Bartolini, P.; Senatra, D.; Ricci, M.; Righini, R.; Barocchi, F.; Torre, R. (2003). "Experimental studies of the ortho-toluidine glass transition". Physical Review E 67 (2). doi:10.1103/PhysRevE.67.021505.
- ↑ Alba-Simionesco, C.; Fan, J.; Angell, C. A. (1999). "Thermodynamic aspects of the glass transition phenomenon. II. Molecular liquids with variable interactions". The Journal of Chemical Physics 110 (11): 5262. doi:10.1063/1.478800.
External links
- MSDS
- o-Toluidine, m-Toluidine, p-Toluidine CDC - NIOSH Pocket to Chemical Hazards