Tin(IV) chloride
| Tin(IV) chloride | |
|---|---|
_chloride.jpg) Anhydrous Tin(IV) chloride |
_chloride_pentahydrate.jpg) Tin(IV) chloride pentahydrate |
-chlorid.svg.png) |
 |
| IUPAC name Tetrachlorostannane | |
| Other names Stannic chloride | |
| Identifiers | |
| CAS number | 7646-78-8 10026-06-9 (pentahydrate) |
| PubChem | 24287 |
| ChemSpider | 22707 |
| EC number | 231-588-9 |
| UN number | 1827 |
| RTECS number | XP8750000 |
| Jmol-3D images | {{#if:Cl[Sn](Cl)(Cl)Cl|Image 1 |
| |
| |
| Properties | |
| Molecular formula | SnCl4 |
| Molar mass | 260.50 g/mol (anhydrous) 350.60 g/mol (pentahydrate) |
| Appearance | colorless fuming liquid |
| Odor | acrid |
| Density | 2.226 g/ml (anhydrous) 2.04 g/cm3 (pentahydrate) |
| Melting point | −33 °C; −27 °F; 240 K |
| Boiling point | 114.15 °C; 237.47 °F; 387.30 K |
| Solubility in water | decomposes (anhydrous) very soluble (pentahydrate) |
| Solubility | soluble in alcohol, benzene, toluene, chloroform, acetone, kerosene, CCl4, methanol, gasoline, CS2 |
| Refractive index (nD) | 1.512 |
| Structure | |
| Crystal structure | monoclinic (P21/c) |
| Hazards | |
| MSDS | ICSC 0953 |
| EU Index | 050-001-00-5 |
| EU classification | Corrosive (C) |
| R-phrases | R34, R52/53 |
| S-phrases | (S1/2), S7/8, S26, S45, S61 |
| NFPA 704 |
 0
3
1
|
| Related compounds | |
| Other anions | Tin(IV) fluoride Tin(IV) bromide Tin(IV) iodide |
| Other cations | Carbon tetrachloride Silicon tetrachloride Germanium tetrachloride Tin(II) chloride Lead(IV) chloride |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Tin(IV) chloride, also known as tin tetrachloride or stannic chloride is a chemical compound with the formula SnCl4. At room temperature it is a colourless liquid, which fumes on contact with air, giving a stinging odor. It was first discovered by Andreas Libavius (1550–1616) and was known as "spiritus fumans libavii".[1]
Preparation
It is prepared from reaction of chlorine gas with elemental tin.
- Sn + 2 Cl2 → SnCl4
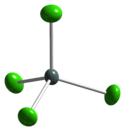
Structure
Tin(IV) chloride solidifies at −33 °C to give monoclinic crystals with the P21/c space group; making it isostructural to solidified SnBr4. Within this the molecules adopt near perfect tetrahedral symmetry with average Sn–Cl distances of 227.9(3) pm.[2]

Reactions
When mixed with a small amount of water a semi-solid crystalline mass of the pentahydrate, SnCl4.5H2O is formed.[1] This was formerly known as butter of tin.[1] This compound has been shown to be best described as [SnCl4(H2O)2].3H2O, consisting of cis-[SnCl4(H2O)2] units linked in chains with three hydrate water molecules.[3] Several lower hydrates have also been characterised.[4]
With hydrochloric acid the complex [SnCl6]2− is formed making the so-called hexachlorostannic acid.[1]
Anhydrous tin(IV) chloride is a strong Lewis acid and complexes with e.g. ammonia, phosphine and phosphorus pentachloride are known.[1] SnCl4 is used in Friedel-Crafts reactions as a catalyst for homogeneous alkylation and cyclisation.[1]
With Grignard reagents tetraalkyltin compounds can be prepared:[5]
- SnCl4 + RMgCl → SnR4 + MgCl2
Uses
Stannic chloride was used as a chemical weapon in World War I, as it formed an irritating (but non-deadly) dense smoke on contact with air: it was substituted for by a mixture of silicon tetrachloride and titanium tetrachloride near the end of the War due to shortages of tin.[6] It is also used in the glass container industry for making an external coating containing tin(IV) oxide which toughens the glass. It is a starting material for organotin compounds.
Stannic chloride is used in chemical reactions with fuming (90%) nitric acid for the selective nitration of activated aromatic rings in the presence of unactivated ones.[7]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
- ↑ Reuter, Hans; Pawlak, Rüdiger (April 2000). "Die Molekül- und Kristallstruktur von Zinn(IV)-chlorid". Zeitschrift für anorganische und allgemeine Chemie (in German) 626 (4): 925–929. doi:10.1002/(SICI)1521-3749(200004)626:4<925::AID-ZAAC925>3.0.CO;2-R.
- ↑ Barnes, John C.; Sampson, Hazel A.; Weakley, Timothy J. R. (1980). "Structures of di-μ-hydroxobis[aquatrichlorotin(IV)]-1,4-dioxane(1/3), di-μ-hydroxobis[aquatrichlorotin(IV)]-1,8-epoxy-p-menthane(1/4), di-m-hydroxobis[aquatribromotin(IV)]-1,8-epoxy-p-menthane(1/4), di-μ-hydroxobis[aquatrichlorotin(IV)], and cis-diaquatetrachlorotin(IV)". J. Chem. Soc., Dalton Trans. (6): 949. doi:10.1039/DT9800000949.
- ↑ Genge, Anthony R. J.; Levason, William; Patel, Rina; Reid, Gillian; Webster, Michael (2004). "Hydrates of tin tetrachloride". Acta Crystallographica Section C Crystal Structure Communications 60 (4): i47–i49. doi:10.1107/S0108270104005633.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0080379419.
- ↑ Fries, Amos A. (2008). Chemical Warfare. Read. pp. 148–49, 407. ISBN 1-4437-3840-9.
- ↑ Thurston, David E.; Murty, Varanasi S.; Langley, David R.; Jones, Gary B. (1990). "O-Debenzylation of a Pyrrolo[2,1-c][1,4]benzodiazepine in the Presence of a Carbinolamine Functionality: Synthesis of DC-81". Synthesis 1990: 81–84. doi:10.1055/s-1990-26795.
External links
| Wikimedia Commons has media related to Tin(IV) chloride. |
| ||||||||