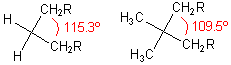Thorpe–Ingold effect
The Thorpe–Ingold effect or gem-dimethyl effect, or angle compression is an effect observed in organic chemistry where increasing the size of two substituents on a tetrahedral center leads to enhanced reactions between parts of the other two substituents. The effect was first reported by Beesley, Thorpe and Ingold in 1915 as part of a study of cyclization reactions.[1]
One illustration of this effect is found in the comparative rates of lactone formation (lactonization) of various 2-hydroxybenzenepropionic acids. The placement of an increasing numbers of methyl groups accelerates the cyclization process.[2]
A common application of this effect is addition of a quaternary carbon (e.g., a gem-dimethyl group) in an alkyl chain to increase the reaction rate and/or equilibrium constant of cyclization reactions. An example of this is an olefin metathesis reaction:[3]
One proposed explanation for this effect is that the increased size of the substituents increases the angle between them. As a result, the angle between the other two substituents decreases. By moving them closer together, reactions between them are accelerated. It is thus a kinetic effect.
The effect also has some thermodynamic contribution as the in silico strain energy decreases on going from cyclobutane to 1-methylcyclobutane and 1,1-dimethylcyclobutane by a value between 8 kcal/mole[4] and 1.5 kcal/mole.[5]
References
- ↑ Beesley, Richard Moore; Ingold, Christopher Kelk; Thorpe, Jocelyn Field (1915). "CXIX.?The formation and stability of spiro-compounds. Part I. Spiro-Compounds from cyclohexane". J. Chem. Soc., Trans. 107: 1080. doi:10.1039/CT9150701080.
- ↑ Michael N. Levine, Ronald T. Raines "Trimethyl lock: a trigger for molecular release in chemistry, biology, and pharmacology (perspective)" Chem. Sci., 2012, volume 3, 2412–2420. doi:10.1039/C2SC20536J
- ↑ Fürstner, A; Langemann, K. (1996). "A Concise Total Synthesis of Dactylol via Ring Closing Metathesis". J. Org. Chem. 61 (25): 8746–8749. doi:10.1021/jo961600c.
- ↑ Conventional Strain Energy in Dimethyl-Substituted Cyclobutane and the gem-Dimethyl Effect Ashley L. Ringer† and David H. Magers J. Org. Chem. 2007, 72, 2533–2537 doi:10.1021/jo0624647
- ↑ The gem-Dimethyl Effect Revisited Steven M. Bachrach J. Org. Chem. 2008, 73, 2466–2468 doi:10.1021/jo702665r