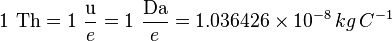Thomson (unit)
| | |
| Unit of | Mass-to-charge ratio |
| Symbol | Th |
| Named after | J. J. Thomson |
The thomson (symbol: Th) is a unit that has appeared infrequently in scientific literature relating to the field of mass spectrometry as a unit of mass-to-charge ratio. The unit was proposed by Cooks and Rockwood[1] naming it in honour of J. J. Thomson who measured the mass-to-charge ratio of electrons and ions.
Definition
The thomson is defined as[2]
where u represents the unified atomic mass unit, Da represents the unit dalton, and e represents the elementary charge which is the electric charge unit in the atomic unit system.
For example, for the ion C7H72+ has an exact mass of 91.0 Da. Its charge number is +2, and hence its charge is 2e. The ion will be observed at 45.5 Th in a mass spectrum.
The thomson allows for negative values for negatively charged ions. For example, the benzoate anion would be observed at m/z 121, but at −121 Th since the charge is −e.
Use
The thomson has been used by some mass spectrometrists, for example Alexander Makarov—the inventor of the Orbitrap—in a scientific poster,[3] papers,[4][5] and (notably) one book.[2] The journal Rapid Communications in Mass Spectrometry (in which the original article appeared) states that "the Thomson (Th) may be used for such purposes as a unit of mass-to-charge ratio although it is not currently approved by IUPAP or IUPAC."[6] Even so, the term has been called "controversial" by RCM's former Editor-in Chief[7] (in a review the Hoffman text cited above[2]). The book, Mass Spectrometry Desk Reference, argues against the use of the thomson.[8] However, the editor-in-chief of the Journal of the Mass Spectrometry Society of Japan has written an editorial in support of the thomson unit.[9]
The thomson is not an SI unit, nor has it been defined by IUPAC.
References
- ↑ Cooks, R. G.; A. L. Rockwood (1991). "The 'Thomson'. A suggested unit for mass spectroscopists". Rapid Communications in Mass Spectrometry 5 (2): 93.
- ↑ 2.0 2.1 2.2 Stroobant, Vincent; Hoffmann, Edmond de; Charette, Jean Joseph (1996). Mass spectrometry: principles and applications. New York: Wiley. ISBN 0-471-96696-7.
- ↑ The Orbitrap: a novel high-performance electrostatic trap (ASMS)
- ↑ Pakenham G, Lango J, Buonarati M, Morin D, Buckpitt A (2002). "Urinary naphthalene mercapturates as biomarkers of exposure and stereoselectivity of naphthalene epoxidation". Drug Metab. Dispos. 30 (3): 247–53. doi:10.1124/dmd.30.3.247. PMID 11854141.
- ↑ Mengel-Jørgensen J, Kirpekar F (2002). "Detection of pseudouridine and other modifications in tRNA by cyanoethylation and MALDI mass spectrometry". Nucleic Acids Res. 30 (23): e135. doi:10.1093/nar/gnf135. PMC 137990. PMID 12466567.
- ↑ "Rapid Communications in Mass Spectrometry Instructions to Authors". Wiley Interscience. Retrieved 2007-12-03.
- ↑ Boyd, Robert K. (4 December 1998). "Book Review: Mass Spectrometry: Principles and Applications. E. de Hoffman, J. Charette and W. Stroobant. Wiley, Chichester 1996. ISBN 0-471-96697-5". Rapid Communications in Mass Spectrometry 11 (8): 948. doi:10.1002/(SICI)1097-0231(199705)11:8<948::AID-RCM2033>3.0.CO;2-I.
- ↑ Sparkman, O. David (2000). Mass spectrometry desk reference. Pittsburgh: Global View Pub. ISBN 0-9660813-2-3.
- ↑ Yoshino, Ken-Ichi (2007). "Comments on Abscissa Labeling of Mass Spectra". Journal of the Mass Spectrometry Society of Japan 55 (1): 51–61. doi:10.5702/massspec.55.51. Retrieved 2007-12-05.