Semicarbazide
| Semicarbazide | |
|---|---|
 | |
| Identifiers | |
| CAS number | 57-56-7 |
| PubChem | 5196 |
| ChemSpider | 5008 |
| KEGG | C02077 |
| ChEBI | CHEBI:28306 |
| ChEMBL | CHEMBL903 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
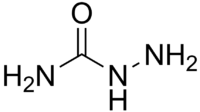
| Molecular formula | H2NNHC(=O)NH2 |
| Molar mass | 75.08 g/mol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
In organic chemistry, semicarbazide is a derivative of urea, where NH2 on one side has been replaced with H2NNH hydrazine, yielding H2NNHC(=O)NH2. A thiosemicarbazide is an analog with a sulfur atom in place of the oxygen atom. Semicarbazone is a ketone form of semicarbazide which is derived by the condensation reaction between a ketone (or aldehyde) and a semicarbazide. Semicarbazide products (semicarbazones and thiosemicarbazones) are known to have an activity of antiviral, antiinfective and antineoplastic through binding to copper or iron in cells. Semicarbazide is used in preparing pharmaceuticals including nitrofuran antibacterials (furazolidone, nitrofurazone, nitrofurantoin) and related compounds. Semicarbazide is used as a detection reagent on thin layer chromatography (TLC). Semicarbazide stains α-keto acids on the TLC plate, which must then be viewed under a UV light to see the results.
-

Thiosemicarbazide