Thiazoline
| Thiazoline | ||
|---|---|---|
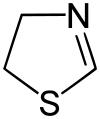 | ||
| IUPAC name 4,5-dihydro-1,3-thiazole | ||
| Other names 4,5-Dihydrothiazole | ||
| Identifiers | ||
| CAS number | 504-79-0 | |
| PubChem | 120269 | |
| Jmol-3D images | Image 1 | |
| ||
| Properties | ||
| Molecular formula | C3H5NS | |
| Appearance | colourless liquid | |
| Boiling point | 135-8 °C | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Thiazoline is a heterocyclic compound containing both sulfur and nitrogen in the ring. Although thiazoline itself is rarely encountered, its derivatives are often bioactive. For example, in a common post-translational modification, cysteine residues are converted into thiazolines.[1]
Synthesis
Thiazolines were first prepared by dialkylation of thioamides.[2] More commonly, they are prepared from derivatives of 2-aminoethanethiol (cysteamine).

Related compounds
Three related classes of C3NS heterocycles are well studied, 1,3-thiazoles (parent: C3H3NS), 1,3-thiazolines (parent: C3H5NS), and 1,3-thiazolidines (parent: C3H7NS). The naming is analogous to the C3N2 heterocycles, imidazoles, imidazolines, and imidazolidines.
Substituted thiazolines
Many molecules contain thiazoline rings, one example being luciferin, the light-emitting molecule in fireflies. The amino acid cysteine is produced industrially from substituted thiazole.[3]
Thiazolines found in nature
In a recent study, thiazolines were identified in nature through an analysis of sesame seed oil. The toasted sesame seed oil was extracted using a Solvent-Assisted Flavor Evaporation technique. The extract was analyzed by GC and GC-MS and a total of 87 components were identified. Amongst these components, 2-ethyl-4-methyl-3-thiazoline and 2-isopropyl-4-methyl-3-thiazoline were identified and confirmed as being present in a natural product for the first time.[4]
References
- ↑ Walsh, Christopher T.; Nolan, Elizabeth M. "Morphing peptide backbones into heterocycles" Proceedings of the National Academy of Sciences USA 2008, volume 105, 5655-5656.doi:10.1073/pnas.0802300105
- ↑ Richard Willstätter, Theodor Wirth "Über Thioformamid" Chem. Ber. 1909, volume 42, 1908-1922. doi:10.1002/cber.19090420267
- ↑ Annie-Claude Gaumont, Mihaela Gulea, and Jocelyne Levillain "Overview of the Chemistry of 2-Thiazolines" Chem. Rev. 2009, volume 109, pp. 1371–1401. doi:10.1021/cr800189z.
- ↑ Agyemang D.; Bardsley K.; Brown S.; Kraut K.; Psota-Kelty L.; Trinnaman L. Identification of 2-Ethyl-4-Methyl-3-Thiazoline and 2-Isopropyl-4-Methyl-3-Thiazoline for the First Time in Nature by the Comprehensive Analysis of Sesame Seed Oil. J. Food Sci. 2011, 76, C385-C391. Doi: http://onlinelibrary.wiley.com/doi/10.1111/j.1750-3841.2011.02071.x/abstract.