Thiamine
| Thiamine | |
|---|---|
 | |
 | |
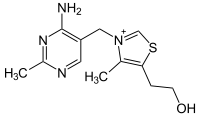
| IUPAC name 2-[3-[(4-Amino-2-methyl-pyrimidin-5-yl)methyl]-4-methyl-thiazol-5-yl] ethanol | |
| Other names Aneurine | |
| Identifiers | |
| CAS number | 59-43-8 |
| PubChem | 6042 |
| ChemSpider | 5819 |
| UNII | X66NSO3N35 |
| EC number | 200-425-3 |
| DrugBank | DB00152 |
| KEGG | C00378 |
| MeSH | Thiamine |
| ChEBI | CHEBI:33283 |
| ChEMBL | CHEMBL1588 |
| ATC code | A11 |
| Beilstein Reference | 3581326 |
| Gmelin Reference | 318226 |
| Jmol-3D images | {{#if:[Cl-].Cc1c(CCO)sc[n+]1Cc1cnc(C)nc1N[Cl-].CC1=C(CCO)SC=[N+]1CC1=CN=C(C)N=C1N[Cl-].n1c(c(cnc1C)C[n+]2c(c(sc2)CCO)C)N|Image 1 Image 2 Image 3 |
| |
| |
| Properties | |
| Molecular formula | C12H17ClN4OS |
| Molar mass | 300.81 g mol−1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Thiamine or thiamin or vitamin B1 (/ˈθaɪ.əmɨn/ THY-ə-min), named as the "thio-vitamine" ("sulfur-containing vitamin") is a water-soluble vitamin of the B complex. First named aneurin for the detrimental neurological effects if not present in the diet, it was eventually assigned the generic descriptor name vitamin B1. Its phosphate derivatives are involved in many cellular processes. The best-characterized form is thiamine pyrophosphate (TPP), a coenzyme in the catabolism of sugars and amino acids. Thiamine is used in the biosynthesis of the neurotransmitter acetylcholine and gamma-aminobutyric acid (GABA). In yeast, TPP is also required in the first step of alcoholic fermentation.
All living organisms use thiamine, but it is synthesized only in bacteria, fungi, and plants. Animals must obtain it from their diet, and thus, for them, it is an essential nutrient. Insufficient intake in birds produces a characteristic polyneuritis. In mammals, deficiency results in Korsakoff's syndrome, optic neuropathy, and a disease called beriberi that affects the peripheral nervous system (polyneuritis) and/or the cardiovascular system. Thiamine deficiency has a potentially fatal outcome if it remains untreated.[1] In less severe cases, nonspecific signs include malaise, weight loss, irritability and confusion.[2]
Chemical properties
Thiamine is a colorless organosulfur compound with a chemical formula C12H17N4OS. Its structure consists of aminopyrimidine and a thiazole ring linked by a methylene bridge. The thiazole is substituted with methyl and hydroxyethyl side chains. Thiamine is soluble in water, methanol, and glycerol and practically insoluble in less polar organic solvents. It is stable at acidic pH, but is unstable in alkaline solutions.[1][3] Thiamine, which is a N-heterocyclic carbene, can be used in place of cyanide as a catalyst for benzoin condensation.[4][5] Thiamine is unstable to heat, but stable during frozen storage. It is unstable when exposed to ultraviolet light[3] and gamma irradiation.[6][7] Thiamine reacts strongly in Maillard-type reactions.[1]
Biosynthesis

Complex thiamine biosynthesis occurs in bacteria, some protozoans, plants, and fungi.[8][9] The thiazole and pyrimidine moieties are bioisynthesized separately and then combined to form ThMP by the action of thiamine-phosphate synthase (EC 2.5.1.3). The biosynthetic pathways may differ among organisms. In E. coli and other enterobacteriaceae, ThMP may be phosphorylated to the cofactor ThDP by a thiamine-phosphate kinase (ThMP + ATP → ThDP + ADP, EC 2.7.4.16). In most bacteria and in eukaryotes, ThMP is hydrolyzed to thiamine, which may then be pyrophosphorylated to ThDP by thiamine diphosphokinase (thiamine + ATP → ThDP + AMP, EC 2.7.6.2).
The biosynthetic pathways are regulated by riboswitches. If there is sufficient thiamine present in the cell then the thiamine binds to the mRNA encoding genes required in the pathway, preventing the translation of the enzymes. If there is no thiamine present then there is no inhibition, and the enzymes required for the biosynthesis are produced. The specific riboswitch, the TPP riboswitch, is the only riboswitch identified in both eukaryotic and prokaryotic organisms.[10]
Nutrition
Occurrence in foods
Thiamine is found in a wide variety of foods at low concentrations. Yeast, yeast extract, and pork are the most highly concentrated sources of thiamine.[citation needed] In general, cereal grains are the most important dietary sources of thiamine, by virtue of their ubiquity. Of these, whole grains contain more thiamine than refined grains, as thiamine is found mostly in the outer layers of the grain and in the germ (which are removed during the refining process). For example, 100 g of whole-wheat flour contains 0.55 mg of thiamine, while 100 g of white flour contains only 0.06 mg of thiamine. In the US, processed flour must be enriched with thiamine mononitrate (along with niacin, ferrous iron, riboflavin, and folic acid) to replace that lost in processing. In Australia, thiamine, folic acid, and iodised salt are added for the same reason.[11] A whole foods diet is therefore recommended for deficiency.
Some other foods rich in thiamine are oatmeal, flax, and sunflower seeds, brown rice, whole grain rye, asparagus, kale, cauliflower, potatoes, oranges, liver (beef, pork, and chicken), and eggs.[2]
Thiamine hydrochloride (Betaxin) is a (when by itself) white, crystalline hygroscopic food-additive used to add a brothy/meaty flavor to gravies or soups. It is a natural intermediary resulting from a thiamine-HCl reaction, which precedes hydrolysis and phosphorylation, before it is finally employed (in the form of TPP) in a number of enzymatic amino, fatty acid, and carbohydrate reactions.[12][13]
Reference Daily Intake and high doses
The RDA in most countries is set at about 1.4 mg. However, tests on female volunteers at daily doses of about 50 mg have claimed an increase in mental acuity.[14] There are no reports available of adverse effects from consumption of excess thiamine by ingestion of food and supplements. Because the data is inadequate for a quantitative risk assessment, no Tolerable Upper Intake Level (UL) can be derived for thiamine.
Antagonists
Thiamine in foods can be degraded in a variety of ways. Sulfites, which are added to foods usually as a preservative,[15] will attack thiamine at the methylene bridge in the structure, cleaving the pyrimidine ring from the thiazole ring.[2] The rate of this reaction is increased under acidic conditions. Thiamine is degraded by thermolabile thiaminases (present in raw fish and shellfish[1]). Some thiaminases are produced by bacteria. Bacterial thiaminases are cell surface enzymes that must dissociate from the membrane before being activated; the dissociation can occur in ruminants under acidotic conditions. Rumen bacteria also reduce sulfate to sulfite, therefore high dietary intakes of sulfate can have thiamine-antagonistic activities.
Plant thiamine antagonists are heat-stable and occur as both the ortho- and para-hydroxyphenols. Some examples of these antagonists are caffeic acid, chlorogenic acid, and tannic acid. These compounds interact with the thiamine to oxidize the thiazole ring, thus rendering it unable to be absorbed. Two flavonoids, quercetin and rutin, have also been implicated as thiamine antagonists.[2]
Absorption and transport
Absorption
Thiamine is released by the action of phosphatase and pyrophosphatase in the upper small intestine. At low concentrations, the process is carrier-mediated, and, at higher concentrations, absorption occurs via passive diffusion. Active transport is greatest in the jejunum and ileum (it is inhibited by alcohol consumption and by folic deficiency).[1] Decline in thiamine absorption occurs at intakes above 5 mg/day.[16] The cells of the intestinal mucosa have thiamine pyrophosphokinase activity, but it is unclear as to whether the enzyme is linked to active absorption. The majority of thiamine present in the intestine is in the pyrophosphorylated form ThDP, but when thiamine arrives on the serosal side of the intestine it is often in the free form. The uptake of thiamine by the mucosal cell is likely coupled in some way to its phosphorylation/dephosphorylation. On the serosal side of the intestine, evidence has shown that discharge of the vitamin by those cells is dependent on Na+-dependent ATPase.[2]
Bound to serum proteins
The majority of thiamine in serum is bound to proteins, mainly albumin. Approximately 90% of total thiamine in blood is in erythrocytes. A specific binding protein called thiamine-binding protein (TBP) has been identified in rat serum and is believed to be a hormone-regulated carrier protein important for tissue distribution of thiamine.[2]
Cellular uptake
Uptake of thiamine by cells of the blood and other tissues occurs via active transport and passive diffusion.[1] The brain requires a much greater amount of thiamine than in other cells of the body. Much of ingested thiamine never reaches the brain because of passive diffusion and the blood brain barrier. About 80% of intracellular thiamine is phosphorylated and most is bound to proteins. In some tissues, thiamine uptake and secretion appears to be mediated by a soluble thiamine transporter that is dependent on Na+ and a transcellular proton gradient.[2]
Tissue distribution
Human storage of thiamine is about 25 to 30 mg, with the greatest concentrations in skeletal muscle, heart, brain, liver, and kidneys. ThMP and free (unphosphorylated) thiamine is present in plasma, milk, cerebrospinal fluid, and, it is presumed, all extracellular fluids. Unlike the highly phosphorylated forms of thiamine, ThMP and free thiamine are capable of crossing cell membranes. Thiamine contents in human tissues are less than those of other species.[2][17]
Excretion
Thiamine and its acid metabolites (2-methyl-4-amino-5-pyrimidine carboxylic acid, 4-methyl-thiazole-5-acetic acid, and thiamine acetic acid) are excreted principally in the urine.[3]
Thiamine phosphate derivatives and function
Thiamine is mainly the transport form of the vitamin, while the active forms are phosphorylated thiamine derivatives. There are five known natural thiamine phosphate derivatives: thiamine monophosphate (ThMP), thiamine diphosphate (ThDP), also sometimes called thiamine pyrophosphate (TPP), thiamine triphosphate (ThTP), and the recently discovered adenosine thiamine triphosphate (AThTP), and adenosine thiamine diphosphate (AThDP).
Thiamine diphosphate
No physiological role is known for ThMP; however, the diphosphate is physiologically relevant. The synthesis of thiamine diphosphate (ThDP), also known as thiamine pyrophosphate (TPP) or cocarboxylase, is catalyzed by an enzyme called thiamine diphosphokinase according to the reaction thiamine + ATP → ThDP + AMP (EC 2.7.6.2). ThDP is a coenzyme for several enzymes that catalyze the transfer of two-carbon units and in particular the dehydrogenation (decarboxylation and subsequent conjugation with coenzyme A) of 2-oxoacids (alpha-keto acids). Examples include:
- Present in most species
- pyruvate dehydrogenase and 2oxoglutarate dehydrogenase (also called α-ketoglutarate dehydrogenase)
- branched-chain α-keto acid dehydrogenase
- 2-hydroxyphytanoyl-CoA lyase
- transketolase
- Present in some species:
- pyruvate decarboxylase (in yeast)
- several additional bacterial enzymes
The enzymes transketolase, pyruvate dehydrogenase (PDH), and 2-oxoglutarate dehydrogenase (OGDH) are all important in carbohydrate metabolism. The cytosolic enzyme transketolase is a key player in the pentose phosphate pathway, a major route for the biosynthesis of the pentose sugars deoxyribose and ribose. The mitochondrial PDH and OGDH are part of biochemical pathways that result in the generation of adenosine triphosphate (ATP), which is a major form of energy for the cell. PDH links glycolysis to the citric acid cycle, while the reaction catalyzed by OGDH is a rate-limiting step in the citric acid cycle. In the nervous system, PDH is also involved in the production of acetylcholine, a neurotransmitter, and for myelin synthesis.[18]
Thiamine triphosphate
Thiamine triphosphate (ThTP) was long considered a specific neuroactive form of thiamine. However, recently it was shown that ThTP exists in bacteria, fungi, plants and animals suggesting a much more general cellular role.[19] In particular in E. coli, it seems to play a role in response to amino acid starvation.[20]
Adenosine thiamine triphosphate
Adenosine thiamine triphosphate (AThTP) or thiaminylated adenosine triphosphate has recently been discovered in Escherichia coli, where it accumulates as a result of carbon starvation.[21] In E. coli, AThTP may account for up to 20% of total thiamine. It also exists in lesser amounts in yeast, roots of higher plants and animal tissue.[22]
Adenosine thiamine diphosphate
Adenosine thiamine diphosphate (AThDP) or thiaminylated adenosine diphosphate exists in small amounts in vertebrate liver, but its role remains unknown.[22]
Deficiency
Thiamine derivatives and thiamine-dependent enzymes are present in all cells of the body, thus a thiamine deficiency would seem to adversely affect all of the organ systems. However, the nervous system is particularly sensitive to thiamine deficiency, because of its dependence on oxidative metabolism.
Thiamine deficiency commonly presents subacutely and can lead to metabolic coma and death. A lack of thiamine can be caused by malnutrition, a diet high in thiaminase-rich foods (raw freshwater fish, raw shellfish, ferns) and/or foods high in anti-thiamine factors (tea, coffee, betel nuts)[23] and by grossly impaired nutritional status associated with chronic diseases, such as alcoholism, gastrointestinal diseases, HIV-AIDS, and persistent vomiting.[24] It is thought that many people with diabetes have a deficiency of thiamine and that this may be linked to some of the complications that can occur.[25][26]
Well-known syndromes caused by thiamine deficiency include beriberi, Wernicke-Korsakoff syndrome, and optic neuropathy.
Alzheimer's Disease
Thiamine deficiency may be detrimental to the cholinergic system and thiamine-dependent enzymes may be altered in Alzheimer's disease; hence thiamine at pharmacologic dosages (3 to 8 g/day thiamine administered orally) may have a mild beneficial effect in dementia of Alzheimer's type.[27] Fursultiamine (TTFD), a derivative of thiamine, had a mild beneficial effect in patients with Alzheimer's disease, as an alternate treatment to large doses of thiamine hydrochloride.[28] Whether thiamine has any effect on Alzheimer's dementia is unclear. A review of double-blinded studies in 2000 found "no evidence that thiamine is a useful treatment for the symptoms of Alzheimer's disease".[29]
Beriberi
Beriberi is a neurological and cardiovascular disease. The three major forms of the disorder are dry beriberi, wet beriberi, and infantile beriberi.[3]
- Dry beriberi is characterized principally by peripheral neuropathy consisting of symmetric impairment of sensory, motor, and reflex functions affecting distal more than proximal limb segments and causing calf muscle tenderness.[24]
However, it has been recently recognized that peripheral neuropathy (tingling or numbness in the extremities) due to thiamine deficiency could also present with axonal neuropathy (partial paralysis or sensory loss). Peripheral neuropathy can present with subacute motor axonal neuropathy mimicking Guillain–Barré syndrome; or as a large fibre proprioceptive central-peripheral axonal neuropathy presenting as a subacute sensory ataxia.[30]
- Wet beriberi is associated with mental confusion, muscular atrophy, edema, tachycardia, cardiomegaly, and congestive heart failure in addition to peripheral neuropathy.[1]
- Infantile beriberi occurs in infants breast-fed by thiamin-deficient mothers (who may show no sign of thiamine deficiency). Infants may manifest cardiac, aphonic, or pseudomeningitic forms of the disorder. Infants with cardiac beriberi frequently exhibit a loud piercing cry, vomiting, and tachycardia.[3] Convulsions are not uncommon, and death may ensue if thiamine is not administered promptly.[24]
Following thiamine treatment, rapid improvement occurs, in general, within 24 hours.[3] Improvements of peripheral neuropathy may require several months of thiamine treatment.[31]
Alcoholic brain disease
Nerve cells and other supporting cells (such as glial cells) of the nervous system require thiamine. Examples of neurologic disorders that are linked to alcohol abuse include Wernicke’s encephalopathy (WE, Wernicke-Korsakoff syndrome) and Korsakoff’s psychosis (alcohol amnestic disorder) as well as varying degrees of cognitive impairment.[32]
Wernicke's encephalopathy is the most frequently encountered manifestation of thiamine deficiency in Western society,[33][34] though it may also occur in patients with impaired nutrition from other causes, such as gastrointestinal disease,[33] those with HIV-AIDS, and with the injudicious administration of parenteral glucose or hyperalimentation without adequate B-vitamin supplementation.[35] This is a striking neuro-psychiatric disorder characterized by paralysis of eye movements, abnormal stance and gait, and markedly deranged mental function.[36]
Korsakoff's syndrome is, in general, considered to occur with deterioration of brain function in patients initially diagnosed with WE.[37] This is an amnestic-confabulatory syndrome characterized by retrograde and anterograde amnesia, impairment of conceptual functions, and decreased spontaneity and initiative.[24]
Alcoholics may have thiamine deficiency because of the following:
- Inadequate nutritional intake: Alcoholics tend to intake less than the recommended amount of thiamine.
- Decreased uptake of thiamine from the GI tract: Active transport of thiamine into enterocytes is disturbed during acute alcohol exposure.
- Liver thiamine stores are reduced due to hepatic steatosis or fibrosis.[38]
- Impaired thiamine utilization: Magnesium, which is required for the binding of thiamine to thiamine-using enzymes within the cell, is also deficient due to chronic alcohol consumption. The inefficient utilization of any thiamine that does reach the cells will further exacerbate the thiamine deficiency.
- Ethanol per se inhibits thiamine transport in the gastrointestinal system and blocks phosphorylation of thiamine to its cofactor form (ThDP).[39]
Following improved nutrition and the removal of alcohol consumption, some impairments linked with thiamine deficiency are reversed, in particular poor brain functionality, although in more severe cases, Wernicke-Korsakoff syndrome leaves permanent damage. (See delirium tremens.)
Optic neuropathy
Optic neuropathy can also occur in thiamine deficiency and is characterized by bilateral visual loss, cecocentral scotomas and impaired colour perception. The ophthalmological findings usually can show a bilateral oedema of the optic disk in the acute phase, followed by a bilateral optic atrophy.[citation needed]
Thiamine deficiency in poultry
As most feedstuffs used in poultry diets contain enough quantities of vitamins to meet the requirements in this species, deficiencies in this vitamin do not occur with commercial diets. This was, at least, the opinion in the 1960s.[40]
Mature chickens show signs 3 weeks after being fed a deficient diet. In young chicks, it can appear before 2 weeks of age.
Onset is sudden in young chicks. There is anorexia and an unsteady gait. Later on, there are locomotor signs, beginning with an apparent paralysis of the flexor of the toes. The characteristic position is called "stargazing", meaning a chick "sitting on its hocks and the head in opisthotonos".
Response to administration of the vitamin is rather quick, occurring a few hours later.[41][42]
Differential diagnosis include riboflavin deficiency and avian encephalomyelitis. In riboflavin deficiency, the "curled toes" is a characteristic symptom. Muscle tremor is typical of avian encephalomyelitis. A therapeutic diagnosis can be tried by supplementing thiamine only in the affected bird. If the animals do not respond in a few hours, thiamine deficiency can be excluded.
Thiamine deficiency in ruminants
Polioencephalomalacia (PEM) is the most common thiamine deficiency disorder in young ruminant and nonruminant animals. Symptoms of PEM include a profuse, but transient, diarrhea, listlessness, circling movements, star gazing or opisthotonus (head drawn back over neck), and muscle tremors.[43] The most common cause is high-carbohydrate feeds, leading to the overgrowth of thiaminase-producing bacteria, but dietary ingestion of thiaminase (e.g., in bracken fern), or inhibition of thiamine absorption by high sulfur intake are also possible.[44] Another cause of PEM is Clostridium sporogenes or Bacillus aneurinolyticus infection. These bacteria produce thiaminases that will cause an acute thiamine deficiency in the affected animal.[45]
Idiopathic paralytic disease in wild birds, fish and mammals
Recently, thiamine deficiency has been identified as the cause of a paralytic disease affecting wild birds in the Baltic Sea area dating back to 1982.[46] In this condition, there is difficulty in keeping the wings folded along the side of the body when resting, loss of the ability to fly and voice, with eventual paralysis of the wings and legs and death. It affects primarily 0.5–1 kg sized birds such as the herring gull (Larus argentatus), Common Starling (Sturnus vulgaris) and Common Eider (Somateria mollissima). Researches noted, "Because the investigated species occupy a wide range of ecological niches and positions in the food web, we are open to the possibility that other animal classes may suffer from thiamine deficiency as well."[46]p. 12006
In the counties of Blekinge and Skåne (south-most Sweden) mass deaths of especially herring gull but also other species has been observed since the early 2000s. More recently, species of other classes seems to be affected. High mortality of salmon (Salmo salar) in the famous river Mörrumsån is reported, and the last years mammals like Eurasian Elk (Alces alces) has suffered death in unusual high number. Lack of thiamine is the common denominator where analysis is done. The County Administrative Board of Blekinge did in April 2012 find the situation so alarming that they asked the Swedish government to set up a closer investigation.[47]
Analysis and diagnostic testing

A positive diagnosis test for thiamine deficiency can be ascertained by measuring the activity of the enzyme transketolase in erythrocytes (Erythrocyte Transketolase Activation Assay). Thiamine, as well as its phosphate derivatives, can also be detected directly in whole blood, tissues, foods, animal feed, and pharmaceutical preparations following the conversion of thiamine to fluorescent thiochrome derivatives (Thiochrome Assay) and separation by high-performance liquid chromatography (HPLC).[48][49][50] In recent reports, a number of Capillary Electrophoresis (CE) techniques and in-capillary enzyme reaction methods have emerged as potential alternative techniques for the determination and monitoring of thiamine in samples.[51] The normal thiamine concentration in EDTA-blood is about 20-100 µg/l.
Genetic diseases
Genetic diseases of thiamine transport are rare but serious. Thiamine responsive megaloblastic anemia (TRMA) with diabetes mellitus and sensorineural deafness[52] is an autosomal recessive disorder caused by mutations in the gene SLC19A2,[53] a high affinity thiamine transporter. TRMA patients do not show signs of systemic thiamine deficiency, suggesting redundancy in the thiamine transport system. This has led to the discovery of a second high-affinity thiamine transporter, SLC19A3.[54][55] Leigh disease (subacute necrotising encephalomyelopathy) is an inherited disorder that affects mostly infants in the first years of life and is invariably fatal. Pathological similarities between Leigh disease and WE led to the hypothesis that the cause was a defect in thiamine metabolism. One of the most consistent findings has been an abnormality of the activation of the pyruvate dehydrogenase complex.[56]
Other disorders in which a putative role for thiamine has been implicated include subacute necrotising encephalomyelopathy, opsoclonic cerebellopathy (a paraneoplastic syndrome), and Nigerian seasonal ataxia. In addition, several inherited disorders of ThDP-dependent enzymes have been reported,[57] which may respond to thiamine treatment.[24]
History
Thiamine was the first of the water-soluble vitamins to be described,[1] leading to the discovery of more such trace compounds essential for survival and to the notion of vitamin.
In 1884, Kanehiro Takaki (1849–1920), a surgeon general in the Japanese navy, rejected the previous germ theory for beriberi and hypothesized that the disease was due to insufficiencies in the diet instead.[58] Switching diet on a navy ship, he discovered that substituting a diet of white rice only, with one also containing barley, meat, milk, bread, and vegetables nearly eliminated beriberi on a 9-month sea voyage. However, Takaki had added many foods to the successful diet and he incorrectly attributed the benefit to increased nitrogen intake, as vitamins were unknown substances at the time. Nor was the Navy convinced of the need for so expensive a program of dietary improvement, and many men continued to die of beriberi, even during the Russo-Japanese war of 1904-5. Not until 1905, after the anti-beriberi factor had been discovered in rice bran (removed by polishing into white rice) and in brown barley rice, was Takaki's experiment rewarded by making him a baron in the Japanese peerage system, after which he was affectionately called "Barley Baron."
The specific connection to grain was made in 1897 by Christiaan Eijkman (1858–1930), a military doctor in the Dutch Indies, discovered that fowl fed on a diet of cooked, polished rice developed paralysis, which could be reversed by discontinuing rice polishing.[59] He attributed beriberi to a nerve poison in the endosperm of rice, from which the outer layers of the grain gave protection to the body. An associate, Gerrit Grijns (1865–1944), correctly interpreted the connection between excessive consumption of polished rice and beriberi in 1901: He concluded that rice contains an essential nutrient in the outer layers of the grain that is removed by polishing.[60]
Eijkman was eventually awarded the Nobel Prize in Physiology and Medicine in 1929, because his observations led to the discovery of vitamins. These compounds were named by Casimir Funk. In 1911, Casimir Funk isolated the antineuritic substance from rice bran that he called a "vitamine" (on account of its containing an amino group). Dutch chemists, Barend Coenraad Petrus Jansen (1884–1962) and his closest collaborator Willem Frederik Donath (1889–1957), went on to isolate and crystallize the active agent in 1926,[61] whose structure was determined by Robert Runnels Williams (1886–1965), a US chemist, in 1934. Thiamine (“sulfur-containing vitamin”) was synthesized in 1936 by the same group.[62]
Thiamine was first named "aneurin" (for anti-neuritic vitamin).[63] Sir Rudolph Peters, in Oxford, introduced thiamine-deprived pigeons as a model for understanding how thiamine deficiency can lead to the pathological-physiological symptoms of beriberi. Indeed, feeding the pigeons upon polished rice leads to an easily recognizable behavior of head retraction, a condition called opisthotonos. If not treated, the animal will die after a few days. Administration of thiamine at the stage of opithotonos will lead to a complete cure of the animal within 30 min. As no morphological modifications were observed in the brain of the pigeons before and after treatment with thiamine, Peeters introduced the concept of biochemical lesion[64]
When Lohman and Schuster (1937) showed that the diphosphorylated thiamine derivative (thiamine diphosphate, ThDP) was a cofactor required for the oxydative decarboxylation of pyruvate,[65] (a reaction now known to be catalyzed by pyruvate dehydrogenase), the mechanism of action of thiamine in the cellular metabolism seemed to be elucidated. At present, this view seems to be oversimplified: Pyruvate dehydrogenase is only one of several enzymes requiring thiamine diphosphate as a cofactor; moreover, other thiamine phosphate derivatives have been discovered since then, and they may also contribute to the symptoms observed during thiamine deficiency.
Finally, the mechanism by which the thiamine moiety of ThDP exerts its coenzyme function by proton substitution on position 2 of the thiazoliumring was elucidated by Ronald Breslow in 1958.[66]
Research
Research in the field mainly concerns the mechanisms by which thiamine deficiency leads to neuronal death in relation to Wernicke Korsakoff Psychosis. Another important theme focuses on understanding of the molecular mechanisms involved in ThDP catalysis. Research has been devoted to the understanding of the possible non-cofactor roles of other derivatives such as ThTP and AThTP.
Mechanism by which thiamine deficiency leads to selective neuronal death
Experimentally induced beriberi polyneuropathy in chickens may be a good model for studying these forms of neuropathy in view of diagnosis and treatment.[67] From studies using rat models, a link between thiamine deficiency and colon carcinogenesis was suggested.[68] Rat model is used also in research of Wernicke's encephalopathy.[69] Thiamine deprived mice are a classic model of systemic oxidative stress, used in research of Alzheimer’s disease.[70]
Catalytic mechanisms in thiamine diphosphate-dependent enzymes
A lot of work is devoted to the understanding of the interplay between ThDP and ThDP-dependent enzymes in catalysis.[71][72]
Non-cofactor roles of thiamine derivatives
Thiamine compounds other than ThDP exist in most cells from many organisms, including bacteria, fungi, plants and animals. Among those compounds are thiamine triphosphate (ThTP) and adenosine thiamine triphosphate (AThTP). They are thought to have non-cofactor roles, though at present it is not known to what extent they participate in the symptoms.[21][22][73][74]
New thiamine derivatives
New thiamine phosphate derivatives continue to be discovered,[21] emphasizing the complexity of thiamine metabolism.
Thiamine derivatives with improved pharmacokinetics may prove effective in alleviating the symptoms of thiamine deficiency and other thiamine-related conditions such as impaired glucose metabolism in diabetes. These compounds include allithiamine, prosultiamine, fursultiamine, benfotiamine, and sulbutiamine, among others.
Persistent carbenes
The production of furoin from furfural is catalyzed by thiamine through a relatively stable carbene (an organic molecule containing unbonded valence electrons pairs at a carbon center). This reaction, studied in 1957 by R. Breslow, was the first evidence for the existence of persistent carbenes.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Mahan, L. K.; Escott-Stump, S., eds. (2000). Krause's food, nutrition, & diet therapy (10th ed.). Philadelphia: W.B. Saunders Company. ISBN 0-7216-7904-8.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Combs, G. F. Jr. (2008). The vitamins: Fundamental Aspects in Nutrition and Health (3rd ed.). Ithaca, NY: Elsevier Academic Press. ISBN 978-0-12-183493-7.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Tanphaichitr V. Thiamin. In: Shils ME, Olsen JA, Shike M et al., editors. Modern Nutrition in Health and Disease. 9th ed. Baltimore: Lippincott Williams & Wilkins; 1999
- ↑ http://www.umsl.edu/~orglab/pdffiles/multistp.pdf
- ↑ http://www.stpaulsschool.org.uk/resource.aspx?id=136714
- ↑ Luczak M, Zeszyty Probi PostepoLc Vauh Roln 1968;80,497; Chem Abstr 1969;71,2267g
- ↑ Syunyakova ZM, Karpova IN, Vop Pitan 1966;25(2),52; Chem Abstr 1966;65,1297b
- ↑ Webb, ME; Marquet, A; Mendel, RR; Rébeillé, F; Smith, AG (2007). "Elucidating biosynthetic pathways for vitamins and cofactors". Nat Prod Rep 24 (5): 988–1008. doi:10.1039/b703105j. PMID 17898894.
- ↑ Begley, TP; Chatterjee, A; Hanes, JW; Hazra, A; Ealick, SE (2008). "Cofactor biosynthesis—still yielding fascinating new biological chemistry". Current Opinion in Chemical Biology 12 (2): 118–125. doi:10.1016/j.cbpa.2008.02.006. PMC 2677635. PMID 18314013.
- ↑ Bocobza, Samuel; Aharoni, Asaph (2008). "Switching the light on plant riboswitches". Trends in Plant Science 13 (10): 526–533. doi:10.1016/j.tplants.2008.07.004. PMID 18778966.
- ↑ Food Standards Australia - Addition of vitamins and minerals to food. Also see Standard 2.1.1 - Cereal Products. The few exceptions include organic wholemeal flour (on the assumption that the wholewheat will have kept more of the nutrients).
- ↑ Skylabs Inc. "Thiamine Hydrochloride Information." 2007.
- ↑ http://www.healthdigest.org/topics/category/1672-thiamine-hydrochloride-dosage-interactions-side-effects-how-to-use
- ↑ Thiamine's Mood-Mending Qualities, Richard N. Podel, Nutrition Science News, January 1999.
- ↑ McGuire, M. and K.A. Beerman. Nutritional Sciences: From Fundamentals to Foods. 2007. California: Thomas Wadsworth.
- ↑ Hayes KC, Hegsted DM. Toxicity of the Vitamins. In: National Research Council (U.S.). Food Protection Committee. Toxicants Occurring Naturally in Foods. 2nd ed. Washington DCL: National Academy Press; 1973.
- ↑ Bettendorff L., Mastrogiacomo F., Kish S. J., and Grisar T. (1996). "Thiamine, thiamine phosphates and their metabolizing enzymes in human brain". J. Neurochem. 66 (1): 250–258. doi:10.1046/j.1471-4159.1996.66010250.x. PMID 8522961.
- ↑ Butterworth RF. Thiamin. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease, 10th ed. Baltimore: Lippincott Williams & Wilkins; 2006
- ↑ Makarchikov AF, Lakaye B, Gulyai IE, Czerniecki J, Coumans B, Wins P, Grisar T and Bettendorff L (2003). "Thiamine triphosphate and thiamine triphosphatase activities: from bacteria to mammals". Cell. Mol. Life Sci 60 (7): 1477–1488. doi:10.1007/s00018-003-3098-4. PMID 12943234.
- ↑ Lakaye B, Wirtzfeld B, Wins P, Grisar T and Bettendorff L (2004). "Thiamine triphosphate, a new signal required for optimal growth of Escherichia coli during amino acid starvation". J. Biol. Chem. 279 (17): 17142–17147. doi:10.1074/jbc.M313569200. PMID 14769791.
- ↑ 21.0 21.1 21.2 Bettendorff L, Wirtzfeld B, Makarchikov AF, Mazzucchelli G, Frédérich M, Gigliobianco T, Gangolf M, De Pauw E, Angenot L and Wins P (2007). "Discovery of a natural thiamine adenine nucleotide". Nature Chemical Biology 3 (4): 211–212. doi:10.1038/nchembio867. PMID 17334376.
- ↑ 22.0 22.1 22.2 Frédérich M., Delvaux D., Gigliobianco T., Gangolf M., Dive G., Mazzucchelli G., Elias B., De Pauw E., Angenot L., Wins P. and Bettendorff L. (2009). "Thiaminylated adenine nucleotides — chemical synthesis, structural characterization and natural occurrence FEBS J". FEBS Journal 276 (12): 3256–3268. doi:10.1111/j.1742-4658.2009.07040.x. PMID 19438713.
- ↑ "Thiamin", Jane Higdon, Micronutrient Information Center, Linus Pauling Institute
- ↑ 24.0 24.1 24.2 24.3 24.4 Butterworth RF. Thiamin. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health and Disease, 10th ed. Baltimore: Lippincott Williams & Wilkins; 2006.
- ↑ Thornalley PJ (2005). "The potential role of thiamine (vitamin B(1)) in diabetic complications". Curr Diabetes Rev 1 (3): 287–98. doi:10.2174/157339905774574383. PMID 18220605.
- ↑ Diabetes problems 'vitamin link', BBC News, 7 August 2007
- ↑ Meador, K; Loring, D; Nichols, M; Zamrini, E; Rivner, M; Posas, H; Thompson, E; Moore, E (1993). "Preliminary findings of high-dose thiamine in dementia of Alzheimer's type". J. Geriatr Psychiatry Neurol. 6 (4): 222–9. PMID 8251051.
- ↑ Mimori, Y; Katsuoka, H; Nakamura, S (1996). "Thiamine therapy in Alzheimer's disease". Metab Brain Dis. 11 (1): 89–94. doi:10.1007/BF02080934. PMID 8815393.
- ↑ Rodríguez-Martín, JL; Qizilbash, N; López-Arrieta, JM (2001). "Thiamine for Alzheimer's disease". In Rodríguez, José-Luis. Cochrane Database Syst Rev. 2 (2): CD001498. doi:10.1002/14651858.CD001498. PMID 11405995.
- ↑ Spinazzi M, Angelini C, Patrini C. Subacute sensory ataxia and optic neuropathy with thiamine deficiency. Nature Reviews Neurology. 2010;6:288-93
- ↑ Maurice V, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and Related Neurologic Disorders Due to Alcoholism and Malnutrition. 2nd ed. Philadelphia: FA Davis, 1989.
- ↑ Martin, PR, Singleton, CK, Hiller-Sturmhofel, S (2003). "The role of thiamine deficiency in alcoholic brain disease". Alcohol Research and Health 27 (2): 134–142. PMID 15303623.
- ↑ 33.0 33.1 Kril JJ (1996). "Neuropathology of thiamine deficiency disorders". Metab Brain Dis 11 (1): 9–17. doi:10.1007/BF02080928. PMID 8815394.
- ↑ For an interesting discussion on thiamine fortification of foods, specifically targetting beer, see "Wernicke's encephalopathy and thiamine fortification of food: time for a new direction?". Medical Journal of Australia.
- ↑ Butterworth RF, Gaudreau C, Vincelette J et al. (1991). "Thiamine deficiency and wernicke's encephalopathy in AIDS". Metab Brain Dis 6 (4): 207–12. doi:10.1007/BF00996920. PMID 1812394.
- ↑ Harper C. (1979). "Wernicke's encephalopathy, a more common disease than realised (a neuropathological study of 51 cases)". J Neurol Neurosurg Psychol 42 (3): 226–231. doi:10.1136/jnnp.42.3.226. PMC 490724. PMID 438830.
- ↑ McCollum EV A History of Nutrition. Cambridge, MA: Riverside Press, Houghton Mifflin; 1957.
- ↑ Butterworth RF (1993). "Pathophysiologic mechanisms responsible for the reversible (thiamine-responsive) and irreversible (thiamine non-responsive) neurological symptoms of Wernicke's encephalopathy". Drug Alcohol Rev 12 (3): 315–22. doi:10.1080/09595239300185371. PMID 16840290.
- ↑ Rindi G, Imarisio L, Patrini C (1986). "Effects of acute and chronic ethanol administration on regional thiamin pyrophosphokinase activity of the rat brain". Biochem Pharmacol 35 (22): 3903–8. doi:10.1016/0006-2952(86)90002-X. PMID 3022743.
- ↑ Merck Veterinary Manual, ed 1967, pp 1440-1441.
- ↑ R.E. Austic and M.L. Scott, Nutritional deficiency diseases, in Diseases of poultry, ed. by M.S. Hofstad, Iowa State University Press, Ames, Iowa, USA ISBN 0-8138-0430-2, p. 50.
- ↑ The disease is described more carefully here: merckvetmanual.com
- ↑ National Research Council. 1996. Nutrient Requirements of Beef Cattle, Seventh Revised Ed. Washington, D.C.: National Academy Press.
- ↑ Polioencephalomalacia: Introduction, Merck Veterinary Manual
- ↑ Polioencephalomacia: Introduction, "ACES Publications"
- ↑ 46.0 46.1 Balk, L; Hägerroth, PA; Akerman, G; Hanson, M; Tjärnlund, U; Hansson, T; Hallgrimsson, GT; Zebühr, Y; Broman, D et al.; Mörner, T.; Sundberg, H. (2009). "Wild birds of declining European species are dying from a thiamine deficiency syndrome". Proc Natl Acad Sci U S A 106 (29): 12001–12006. doi:10.1073/pnas.0902903106. PMC 2715476. PMID 19597145.
- ↑ Blekinge län, Länsstyrelsen (2013). 2012-04-15 500-1380-13 Förhöjd dödlighet hos fågel, lax og älg.
- ↑ Bettendorff, L, Peeters, M., Jouan, C., Wins, P., Schoffeniels, E. (1991). "Determination of thiamin and its phosphate esters in cultured neurons and astrocytes using an ion-pair reversed-phase high-performance liquid chromatographic method". Anal. Biochem 198 (1): 52–59. doi:10.1016/0003-2697(91)90505-N. PMID 1789432.
- ↑ Losa, R, Sierra, MI, Fernández, A, Blanco, D, Buesa, JM. (2005). "Determination of thiamine and its phosphorylated forms in human plasma, erythrocytes and urine by HPLC and fluorescence detection: a preliminary study on cancer patients". J Pharm Biomed Anal 37 (5): 1025–1029. doi:10.1016/j.jpba.2004.08.038. PMID 15862682.
- ↑ Lu, J, Frank, EL. (May 2008). "Rapid HPLC measurement of thiamine and its phosphate esters in whole blood". Clin Chem. 54 (5): 901–906. doi:10.1373/clinchem.2007.099077. PMID 18356241.
- ↑ Shabangi, M, Sutton, JA. (2005). "Separation of thiamin and its phosphate esters by capillary zone electrophoresis and its application to the analysis of water-soluble vitamins". Journal of Pharmaceutical and Biomedical Analysis 38 (1): 66–71. doi:10.1016/j.jpba.2004.11.061. PMID 15907621.
- ↑ Slater, PV (1978). "Thiamine Responsive Megaloblastic Anemia with severe diabetes mellitus and sensorineural deafness (TRMA)". The Australian nurses' journal 7 (11): 40–3. PMID 249270.
- ↑ Kopriva, V; Bilkovic, R; Licko, T (Dec 1977). "Tumours of the small intestine (author's transl)". Ceskoslovenska gastroenterologie a vyziva 31 (8): 549–53. ISSN 0009-0565. PMID 603941.
- ↑ Beissel, J (Dec 1977). "The role of right catheterization in valvular prosthesis surveillance (author's transl)". Annales de cardiologie et d'angéiologie 26 (6): 587–9. ISSN 0003-3928. PMID 606152.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 249270
- ↑ Butterworth RF. Pyruvate dehydrogenase deficiency disorders. In: McCandless DW, ed. Cerebral Energy Metabolism and Metabolic Encephalopathy. Plenum Publishing Corp.; 1985.
- ↑ Blass JP. Inborn errors of pyruvate metabolism. In: Stanbury JB, Wyngaarden JB, Frederckson DS et al., eds. Metabolic Basis of Inherited Disease. 5th ed. New York: McGraw-Hill, 1983.
- ↑ McCollum EV. A History of Nutrition. Cambridge, Mass.: Riverside Press, Houghton Mifflin; 1957.
- ↑ Eijkman, C. (1897). "Eine Beriberiähnliche Krankheit der Hühner". Virchows Arch. Pathol. Anat. 148 (3): 523. doi:10.1007/BF01937576.
- ↑ Grijns, G. (1901). "Over polyneuritis gallinarum". I. Geneesk. Tijdscht. Ned. Ind. 43: 3–110.
- ↑ Jansen, B.C.P.; Donath, W.F. (1926). "On the isolation of antiberiberi vitamin". Proc. Kon. Ned. Akad. Wet. 29: 1390–1400.
- ↑ Williams, R.R.; Cline, J.K. (1936). "Synthesis of vitamin B1". J. Am. Chem. Soc. 58 (8): 1504–1505. doi:10.1021/ja01299a505.
- ↑ Carpenter KJ. Beriberi, white rice, and vitamin B: a disease, a cause, and a cure. Berkeley, CA: University of California Press; 2000
- ↑ Peters, R.A. (1936). "The biochemical lesion in vitamin B1deficiency. Application of modern biochemical analysis in its diagnosis". Lancet 1 (5882): 1161–1164. doi:10.1016/S0140-6736(01)28025-8.
- ↑ Lohmann, K.; Schuster, P. (1937). "Untersuchungen über die Cocarboxylase". Biochem. Z. 294: 188–214.
- ↑ Breslow R (1958). "On the mechanism of thiamine action. IV.1 Evidence from studies on model systems". J Am Chem Soc 80 (14): 3719–3726. doi:10.1021/ja01547a064.
- ↑ Djoenaidi W, Notermans SL, Gabreëls-Festen AA, Lilisantoso AH, Sudanawidjaja A (1995). "Experimentally induced beriberi polyneuropathy in chickens". Electromyogr Clin Neurophysiol 35 (1): 53–60. PMID 7737017.
- ↑ Bruce WR, Furrer R, Shangari N, O’Brien PJ, Medline A, Wang Y (2003). "Marginal dietary thiamin deficiency induces the formation of colonic aberrant crypt foci (ACF) in rats". Cancer Lett 202 (2): 125–129. doi:10.1016/j.canlet.2003.08.005. PMID 14643441.
- ↑ Langlais PJ (1995). "Pathogenesis of diencephalic lesions in an experimental model of Wernicke's encephalopathy". Metab Brain Dis 10 (1): 31–44. doi:10.1007/BF01991781. PMID 7596327.
- ↑ Frederikse PH, Zigler SJ Jr, Farnsworth PN, Carper DA (2000). "Prion protein expression in mammalian lenses". Curr Eye Res 20 (2): 137–43. doi:10.1076/0271-3683(200002)20:2;1-D;FT137. PMID 10617916.
- ↑ Kale, S; Ulas, G; Song, J; Brudvig, GW; Furey, W; Jordan, F (2008). "Efficient coupling of catalysis and dynamics in the E1 component of Escherichia coli pyruvate dehydrogenase multienzyme complex". Proc Natl Acad Sci U S A 105 (4): 1158–1163. doi:10.1073/pnas.0709328105. PMC 2234108. PMID 18216265.
- ↑ Kluger, R; Tittmann, K (2008). "Thiamin diphosphate catalysis: enzymic and nonenzymic covalent intermediates". Chem Rev 108 (6): 1797–1833. doi:10.1021/cr068444m. PMID 18491870.
- ↑ Makarchikov, AF; Lakaye, B; Gulyai, IE; Czerniecki, J; Coumans, B; Wins, P; Grisar, T; Bettendorff, L (2003). "Thiamine triphosphate and thiamine triphosphatase activities: from bacteria to mammals". Cell Mol Life Sci 60 (7): 1477–1488. doi:10.1007/s00018-003-3098-4. PMID 12943234.
- ↑ Bettendorff L. and Wins P. (2009). "Thiamin diphosphate in biological chemistry : new aspects of thiamin metabolism, especially triphosphate derivatives acting other than as cofactors". FEBS J. 276 (11): 2917–2925. doi:10.1111/j.1742-4658.2009.07017.x. PMID 19490098.
External links
- "Thiamine"
- "Branched-Chain Amino Acid Metabolism" at ncbi.nlm.nih.gov
- Thiamin deficiency in poultry
- Type of vitamin B1 could treat common cause of blindness
- Thiamin bound to proteins in the PDB
| |||||||||||||||||||||||||||||||||||||||