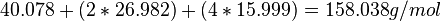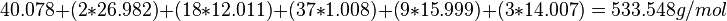Thermogravimetric analysis
| Acronym | TGA |
|---|---|
| Classification |
|
| Other techniques | |
| Related |
Isothermal microcalorimetry Differential scanning calorimetry Dynamic mechanical analysis Thermomechanical analysis Differential thermal analysis Dielectric thermal analysis |
Thermogravimetric analysis or thermal gravimetric analysis (TGA) is a method of thermal analysis in which changes in physical and chemical properties of materials are measured as a function of increasing temperature (with constant heating rate), or as a function of time (with constant temperature and/or constant mass loss).[1] TGA can provide information about physical phenomena, such as second-order phase transitions, including vaporization, sublimation, absorption, adsorption, and desorption. Likewise, TGA can provide information about chemical phenomena including chemisorptions, desolvation (especially dehydration), decomposition, and solid-gas reactions (e.g., oxidation or reduction).[1]
TGA is commonly used to determine selected characteristics of materials that exhibit either mass loss or gain due to decomposition, oxidation, or loss of volatiles (such as moisture). Common applications of TGA are (1) materials characterization through analysis of characteristic decomposition patterns, (2) studies of degradation mechanisms and reaction kinetics, (3) determination of organic content in a sample, and (4) determination of inorganic (e.g. ash) content in a sample, which may be useful for corroborating predicted material structures or simply used as a chemical analysis. It is an especially useful technique for the study of polymeric materials, including thermoplastics, thermosets, elastomers, composites, plastic films, fibers, coatings and paints. Discussion of the TGA apparatus, methods, and trace analysis will be elaborated upon below. Thermal stability, oxidation, and combustion, all of which are possible interpretations of TGA traces, will also be discussed.
Instrumental apparatus
Thermogravimetric analysis relies on a high degree of precision in three measurements: mass change, temperature, and temperature change. Therefore, the basic instrumental requirements for TGA are a precision balance with a pan loaded with the sample, and a programmable furnace. The furnace can be programmed either for a constant heating rate, or for heating to acquire a constant mass loss with time.
Though a constant heating rate is more common, a constant mass loss rate can illuminate specific reaction kinetics. For example, the kinetic parameters of the carbonization of polyvinyl butyral were found using a constant mass loss rate of 0.2 wt %/min.[2] Regardless of the furnace programming, the sample is placed in a small, electrically heated furnace equipped with a thermocouple to monitor accurate measurements of the temperature by comparing its voltage output with that of the voltage-versus-temperature table stored in the computer’s memory. A reference sample may be placed on another balance in a separate chamber. The atmosphere in the sample chamber may be purged with an inert gas to prevent oxidation or other undesired reactions. A different process using a quartz crystal microbalance has been devised for measuring smaller samples on the order of a microgram (versus milligram with conventional TGA).[3] Figure 1 provides schematic diagrams of a typical TGA instrument.
Methods
The TGA instrument continuously weighs a sample as it is heated to temperatures of up to 2000°C for coupling with FTIR and Mass spectrometry gas analysis. As the temperature increases, various components of the sample are decomposed and the weight percentage of each resulting mass change can be measured. Results are plotted with temperature on the X-axis and mass loss on the Y-axis. The data can be adjusted using curve smoothing and first derivatives are often also plotted to determine points of inflection for more in-depth interpretations (see discussion on Trace Analysis).
Trace analysis
If the identity of the product after heating is known, then the ceramic yield can be found from analysis of the ash content (see discussion below). By taking the weight of the known product and dividing it by the initial mass of the starting material, the mass percentage of all inclusions can be found. Knowing the mass of the starting material and the total mass of inclusions, such as ligands, structural defects, or side-products of reaction, which are liberated upon heating, the stoichiometric ratio can be used to calculate the percent mass of the substance in a sample. The results from thermogravimetric analysis may be presented by (1) mass versus temperature (or time) curves, referred to as the Thermogravimetric curve, or (2) rate of mass loss versus temperature curve, referred to as the differential Thermogravimetric curve, see Figure 2. Though this is by no means an exhaustive list, simple thermogravimetric curves may contain the following features:
- A horizontal portion, or plateau that indicates constant sample weight (Regions A and C in Figure 2)
- A curved portion; the steepness of the curve indicates the rate of mass loss (Region B in Figure 2)
- An inflection (at which
 is a minimum, but not zero)
is a minimum, but not zero)
Certain features in the TGA curve that are not readily seen can be more clearly discerned in the first derivative TGA curve. For example, any change in the rate of weight loss can immediately be seen in the first derivative TGA curve as a trough, or as a shoulder or tail to the peak, indicating two consecutive or overlapping reactions. Differential TGA curves also can show considerable similarity to differential thermal analysis (DTA) curves, which can permit easy comparisons to be made.[1]
Ceramic yield
Ceramic yield is defined as the mass percent of starting material found in the end product. From this, stoichiometry can then be used to calculate the percent mass of the substance in the sample.
Metal aluminates (MAl2O4) are an important type of mixed-cation oxide ceramics that have many applications.[4] The metal aluminate CaAl2O4 is used in the cement industry as a hydraulic material.[4] The TGA of the precursor to calcium aluminate CaAl2C18H37O9N3 is shown in Figure 3.[4] The formation of CaAl2O4 occurs during the thermogravimetric analysis. This is how the theoretical ceramic yield is calculated for this example:
(1) Calculate molecular weight of CaAl2O4:
(2) Calculate molecular weight of CaAl2C18H37O9N3:
(3) Calculate the percentage that CaAl2O4 is of CaAl2C18H37O9N3:
Therefore, the theoretical ceramic yield for the thermogravimetric analysis of CaAl2C18H37O9N3 is 29.6%. This correlates well with the experimentally determined ceramic yield of 28.9%.
As another example of calculating theoretical ceramic yield, the TGA of calcium oxalate monohydrate is shown in Figure 4. Using the same process detailed above, the theoretical ceramic yield can be calculated: the formula weight of calcium oxalate monohydrate is 146 g/mol. The final ceramic product is CaO, with a formula weight of 56 g/mol. The theoretical ceramic yield is therefore 38.4%. The actual yield from the TGA was found to be 39.75%. Some reasons for discrepancies between the theoretical and actual yields are trapped CO2 and the formation of metal carbides.
In the TGA trace of calcium oxalate monohydrate, the first mass loss corresponds to loss of water of hydration. The second mass loss corresponds to decomposition of dehydrated calcium oxalate to calcium carbonate and carbon monoxide and carbon dioxide. The last mass loss is due to the decomposition of calcium carbonate to calcium oxide and carbon dioxide.
Figure 5 shows thermograms of four different chloro-polymers: (a) polyvinyl chloride, (b) chlorinated polyvinyl chloride, (c) chlorinated rubber, and (d) polyvinylidene chloride.[5] There are two stages of degradation in these four polymers. The first stage is the loss of hydrogen chloride, and is complete around 250°C. This first step occurs at lower temperatures for the polymers containing more chlorine (chlorinated polyvinyl chloride, chlorinated rubber, and polyvinylidene chloride), implying that these chloride groupings are less stable than in polyvinyl chloride.[5]
The second stage is the carbonization of the polymer, and takes place between 250°C and 500°C. This is seen by the large loss of mass between 250°C and 500°C. Tar and simple gases, such as hydrogen and methane, are evolved and the carbon that remains loses very little mass between 500°C and 900°C. In this second stage, the higher the chlorine content of the polymer, the lower the yield of tar. This is because chlorine is able to remove hydrogen, which would otherwise be used in the compounds that form tar.[5]
Thermal stability
TGA can be used to evaluate the thermal stability of a material. In a desired temperature range, if a species is thermally stable, there will be no observed mass change. Negligible mass loss corresponds to little or no slope in the TGA trace. TGA also gives the upper use temperature of a material. Beyond this temperature the material will begin to degrade.
TGA has a wide variety of applications, including analysis of ceramics and thermally stable polymers. Ceramics usually melt before they decompose as they are thermally stable over a large temperature range, thus TGA is mainly used to investigate the thermal stability of polymers. Most polymers melt or degrade before 200°C. However, there is a class of thermally stable polymers that are able to withstand temperatures of at least 300°C in air and 500°C in inert gases without structural changes or strength loss, which can be analyzed by TGA.[6] For example, the polyimide Kapton® loses less than 10% mass when held in 400°C air for 100 hours.[6]
Figure 6 compares various high performance fibers using TGA as an evaluation of thermal stability. From the TGA, polyoxazole (PBO) has the highest thermal stability of the four fibers as it is stable up to ca. 500°C. Ultra-high-molecular-weight polyethylene (UHMW-PE) has the lowest thermal stability, as it begins to degrade around 200°C. Often the onset of mass loss is seen more prominently in the first derivative of the mass loss curve, as evidenced by Figure 2. High performance fibers used in bulletproof vests must remain strong enough mechanically so as to protect the user from incoming projectiles. The thermal and photochemical degradation of the fibers causes the mechanical properties of the vests to decrease, effectively rendering the armor useless. Thus, thermal stability is a key property when designing these vests.[7]
Three ways a material can lose mass during heating are through chemical reactions, the release of adsorbed species, and decomposition. All of these indicate that the material is no longer thermally stable. Out of the four fibers shown in Figure 6, only Terlon shows loss of adsorbed species, most likely water, as the mass loss occurs after 100°C. Because the TGA is performed in air, oxygen reacts with the organic fibers which eventually degrade completely, evidenced by the 100% mass loss. It is important to link thermal stability to the gas in which the TGA is performed. PBO, which completely decomposes when heated in air, retains ~60% mass when heated in N2.[8] Thus, PBO is thermally stable in nitrogen up to 630°C, whereas in air, PBO has almost completely decomposed at that temperature.
Oxidation processes
Oxidative mass losses are the most common observable losses in TGA.[9] Figure 7 shows the mass gain vs. temperature for three copper alloys. All of the mass gain of these alloys is due to oxidation. The histogram also includes the mass gain of copper alone.[10] The composition of the GR-84 alloy is 8 wt.% Cu, 4 wt.% Cr and the remainder is Nb. The composition of the GC-15 alloy is copper with 0.15wt.% Al. The composition of the NAR-Z alloy is Cu-3 wt.% Al-0.5 wt.% Zr. This last alloy was the liner of the main engine of the space shuttle in 2005.[10]
Studying the resistance to oxidation in copper alloys is very important. For example, NASA (National Aeronautics and Space Administration) is conducting research on advanced copper alloys for their possible use in combustion engines. However, oxidative degradation can occur in these alloys as copper oxides form in atmospheres that are rich in oxygen. Resistance to oxidation is very important because NASA wants to be able to reuse shuttle materials. TGA can be used to study the static oxidation of materials such as these for practical use.
Some researchers have been studying ways in which to protect certain oligomers or polymers from oxidation processes. One example is inserting an oligomer into a multiblock copolymer.[11] The TGA traces of both the oligomer and the oligomer/multiblock copolymer in N2 and in air are shown in Figure 8.[11] When the TGAs were run under a nitrogen atmosphere (Figure 8a and b), there is no oxidation of the substrate. When the TGA of the oligomer was run under air (Figure 8c), an oxidation process can be seen between 200°C-350°C. This process is not seen for the oligomer/multiblock copolymer (Figure 8d). The authors of this paper explained this disappearance by suggesting that the oxidative process involved hydroxyl end groups on the oligomer. The encasing of the oligomer by the multiblock copolymer prevented this from happening.[11]
Combustion
Combustion during TG analysis is identifiable by distinct traces made in the TGA thermograms produced. One interesting example occurs with samples of as-produced unpurified carbon nanotubes that have a large amount of metal catalyst present (See Figure 9). Due to combustion, a TGA trace can deviate from the normal form of a well-behaved function. This phenomenon arises from a rapid temperature change. When the weight and temperature are plotted versus time, a dramatic slope change in the first derivative plot is concurrent with the mass loss of the sample and the sudden increase in temperature seen by the thermocouple. The mass loss could be the result of particles of smoke released from burning caused by inconsistencies in the material itself, beyond the oxidation of carbon due to poorly controlled weight loss.
References
- ↑ 1.0 1.1 1.2 Coats, A. W.; Redfern, J. P. (1963). "Thermogravimetric Analysis: A Review". Analyst 88: 906–924. Bibcode:1963Ana....88..906C. doi:10.1039/AN9638800906.
- ↑ Tikhonov, N. A.; Arkhangelsky, I. V.; Belyaev, S. S.; Matveev, A. T. (2009). "Carbonization of polymeric nonwoven materials". Thermochimica Acta 486: 66–70. doi:10.1016/j.tca.2008.12.020.
- ↑ "Thermogravimetric Analysis".
- ↑ 4.0 4.1 4.2 Narayanan, R.; Laine, R. M. (1997). "Synthesis and Characterization of Precursors for Group II Metal Aluminates". Appl. Organomet. Chem. 11: 919–927. doi:10.1002/(SICI)1099-0739(199710/11)11:10/11<919::AID-AOC666>3.0.CO;2-Z.
- ↑ 5.0 5.1 5.2 Gilbert, J. B.; Kipling, J. J.; McEnaney, B.; Sherwood, J. N. (1962). "Carbonization of Polymers I - Thermogravimetric Analysis". Polymer 3: 1–10.
- ↑ 6.0 6.1 Marvel, C. S. (1972). "Synthesis of Thermally Stable Polymers". Ft. Belvoir: Defense Technical Information Center.
- ↑ Liu, X.; Yu, W. (2006). "Evaluating the Thermal Stability of High Performance Fibers by TGA". Journal of Applied Polymer Science 99: 937–944. doi:10.1002/app.22305.
- ↑ Tao, Z.; Jin, J.; Yang, S.; Hu, D.; Li, G.; Jiang, J. (2009). "Synthesis and Characterization of Fluorinated PBO with High Thermal Stability and Low Dielectric Constant". Journal of Macromolecular Science, Part B 48: 1114–1124. doi:10.1080/00222340903041244.
- ↑ Voitovich, V. B.; Lavrenko, V. A.; Voitovich, R. F.; Golovko, E. I. (1994). "The Effect of Purity on High-Temperature Oxidation of Zirconium". Oxidation of Metals 42: 223–237. doi:10.1007/BF01052024.
- ↑ 10.0 10.1 Ogbuji, L. U.; Humphrey, D. L. (2003). "Comparison of the Oxidation Rates of Some New Copper Alloys". Oxidation of Metals 60: 271–291. doi:10.1023/A:1026019202691.
- ↑ 11.0 11.1 11.2 D'Antone, S.; Bignotti, F.; Sartore, L.; D’Amore, A.; Spagnoli, G.; Penco, M. (2001). "Thermogravimetric investigation of two classes of block copolymers based on poly(lactic-glycolic acid) and poly(ε-caprolactone) or poly(ethylene glycol)". Polymer Degradation and Stability 74: 119–124. doi:10.1016/S0141-3910(01)00110-0.