Theogallin
From Wikipedia, the free encyclopedia
| Theogallin | |
|---|---|
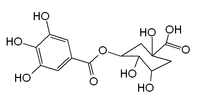 | |
| IUPAC name (1S,3R,4R,5R)-1,3,4-trihydroxy-5-(3,4,5-trihydroxybenzoyl)oxycyclohexane-1-carboxylic acid | |
| Other names 3-O-Galloylquinic acid | |
| Identifiers | |
| CAS number | 17365-11-6 |
| PubChem | 442988 |
| ChemSpider | 391291 |
| ChEBI | CHEBI:9522 |
| Jmol-3D images | {{#if:O=C(O)[C@]2(O)C[C@@H](O)[C@@H](O)[C@H](OC(=O)c1cc(O)c(O)c(O)c1)C2|Image 1 |
| |
| |
| Properties | |
| Molecular formula | C14H16O10 |
| Molar mass | 344.27 g/mol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Theogallin is a trihydroxybenzoic acid glycoside, a type of polyphenolic compound found in tea[1] where it has been characterised as an umami enhancing compound.[2] The compound can also be found in Arbutus unedo fruits.[3]
In rats, theogallin, or its metabolite quinic acid, can move through the blood–brain barrier and can have cognition enhancing activities.[4]
References
- ↑ Cartwright, R. A.; Roberts, E. A. H. (1954). "Theogallin, a polyphenol occurring in tea". Journal of the Science of Food and Agriculture 5 (12): 593. doi:10.1002/jsfa.2740051207.
- ↑ Kaneko, S; Kumazawa, K; Masuda, H; Henze, A; Hofmann, T (2006). "Sensory and structural characterisation of an umami enhancing compound in green tea (mat-cha)". Flavour Science - Recent Advances and Trends. Developments in Food Science 43. p. 181. doi:10.1016/S0167-4501(06)80043-9. ISBN 978-0-444-52742-4.
- ↑ Pawlowska, A.M., De Leo, M., Braca, A. (2006). "Phenolics of Arbutus unedo L. (Ericaceae) fruits: identification of anthocyanins and gallic acid derivatives". J. Agric. Food Chem. 54 (26): 10234–8. doi:10.1021/jf062230o. PMID 17177565.
- ↑ Dimpfel, Wilfried; Kler, Adolf; Kriesl, Erwin; Lehnfeld, Romanus (2007). "Theogallin and l-theanine as active ingredients in decaffeinated green tea extract: II. Characterization in the freely moving rat by means of quantitative field potential analysis". Journal of Pharmacy and Pharmacology 59 (10): 1397–403. doi:10.1211/jpp.59.10.0010. PMID 17910815.
| ||||||||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.