Thallium azide
From Wikipedia, the free encyclopedia
| Thallium azide | ||
|---|---|---|
 | ||
| Other names thallium azide | ||
| Identifiers | ||
| PubChem | 22764821 | |
| ChemSpider | 15368504 | |
| Jmol-3D images | {{#if:[Tl+].[N-]=[N+]=[N-]|Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | TlN3 | |
| Molar mass | 246.4035 | |
| Appearance | yellow-brown | |
| Solubility in water | insoluble | |
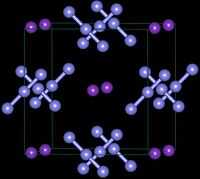
| Structure | ||
| Crystal structure | Tetragonal, tI16 [1] | |
| Space group | I4/mcm, No. 140 | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Thallium azide, TlN3, is a yellow-brown crystalline solid poorly soluble in water. Although it is not nearly as sensitive to shock or friction as lead azide, it can easily be detonated by a flame or spark. It can be stored safely dry in a closed non-metallic container.
Preparation
Thallium azide can be prepared by dissolving thallium(I) sulfate in water, and adding sodium azide solution. Thallium azide will precipitate; the yield can be maximized by cooling in a freezer.
Safety
All thallium compounds are poisonous and should be handled with care; avoid breathing any dust or fumes.
References
- ↑ Mauer F.A., Hubbard C.R., Hahn T.A. (1973). "Thermal expansion and low temperature phase transition of thallous azide". J. Chem. Phys. 59 (7): 3770–3776. doi:10.1063/1.1680549.
| |||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.