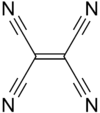
Tetracyanoethylene
| Tetracyanoethylene | |
|---|---|
 |
 |
| IUPAC name ethenetetracarbonitrile | |
| Other names TCNE | |
| Identifiers | |
| CAS number | 670-54-2 |
| PubChem | 12635 |
| ChemSpider | 12114 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C6N4 |
| Molar mass | 128.09 g/mol |
| Density | g/cm3 |
| Melting point | 199 °C |
| Boiling point | 130-140 °C, 0.1 mm Hg (sublimes)[1] |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Tetracyanoethylene (TCNE), more correctly ethenetetracarbonitrile, is a clear colored organic compound consisting of ethylene with the four hydrogen atom replaced with cyano groups. It is an important member of the cyanocarbons.
Synthesis and reactions
TCNE is prepared by brominating malononitrile in the presence of potassium bromide to give the KBr-complex, and dehalogenating with copper.[1]
Oxidation of TCNE with hydrogen peroxide gives the corresponding epoxide, which has unusual properties.[2]
Redox chemistry
TCNE is often used as an electron acceptor. Cyano groups have low energy π* orbitals, and the presence of four such groups, with their π systems linked (conjugated) to the central C=C double bond, gives rise to an excellent acceptor. Thus, treatment of TCNE with iodide salts gives the radical anion:
- C2(CN)4 + I- → [C2(CN)4]- + 0.5 I2
Because of its planarity and its ability to accept electrons, TCNE has been used to prepare numerous organic superconductors, usually by serving as a single electron oxidant of an organic electron donor. Such charge-transfer salts are sometimes called Bechgaard salt.
Safety
TCNE hydrolyzes in moist air to give hydrogen cyanide and should be handled accordingly.[1]
References
- ↑ 1.0 1.1 1.2 Carboni, R. A. (1963), "Tetracyanoethylene", Org. Synth.; Coll. Vol. 4: 877
- ↑ Linn, W. J. (1973), "Tetracyanoethylene Oxide", Org. Synth.; Coll. Vol. 5: 1007