Terpinene
| Terpinenes | |
|---|---|
 α-Terpinene |
 β-Terpinene |
 γ-Terpinene |
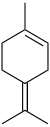 δ-Terpinene (terpinolene) |
| IUPAC name α: 4-Methyl-1-(1-methylethyl)-1,3-cyclohexadiene | |
| Identifiers | |
| CAS number | 99-86-5 (α) |
| ChemSpider | 60205 |
| ChEBI | CHEBI:59159 |
| Jmol-3D images | Image 1 Image 2 Image 3 Image 4 |
| |
| |
| Properties | |
| Molecular formula | C10H16 |
| Molar mass | 136.23 g mol−1 |
| Density | α: 0.8375 g/cm3 β: 0.838 g/cm3 γ: 0.853 g/cm3 |
| Melting point | α: 60-61 °C |
| Boiling point | α: 173.5-174.8 °C β: 173-174 °C γ: 183 °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
The terpinenes are a group of isomeric hydrocarbons that are classified as terpenes. They each have the same molecular formula and carbon framework, but they differ in the position of carbon-carbon double bonds. α-Terpinene has been isolated from cardamom and marjoram oils, and from other natural sources. β-Terpinene has no known natural source, but has been prepared synthetically from sabinene. γ-Terpinene and δ-terpinene (also known as terpinolene) are natural and have been isolated from a variety of plant sources.
Biosynthesis of α-terpinene
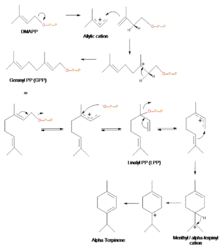
The biosynthesis of α-terpinene and other terpenoids occurs via the mevalonate pathway because its starting reactant, dimethylallyl pyrophosphate (DMAPP), is derived from mevalonic acid. Though α-terpinene is commonly considered a perfume and flavoring chemical and therefore used in the cosmetics and food industries, its use both in the pharmaceutical and electronics semi-conductor manufacturing industries have also proven to be valuable.
Geranyl pyrophosphate (GPP) is produced from the reaction of a resonance-stable allylic cation, formed from the loss of the diphosphate group from DMAPP, and isopentenyl pyrophosphate (IPP), and a subsequent the loss of a proton. GPP then loses the diphosphate group to form the resonance-stable geranyl cation. The reintroduction of the diphosphate group to the cation produces GPP isomer, known as linalyl pyrophosphate (LPP). LPP then forms a resonance-stable cation by losing its diphosphate group. Cyclization is then completed thanks to this more favorable stereochemistry of the LPP cation, now yielding the menthyl/α-terpinyl cation. Finally, a 1,2-hydride shift via a Wagner-Meerwein rearrangement produces the terpinen-4-yl cation. It is the loss of a hydrogen from this cation that generates α-terpinene.
List of the plants that contain one of the chemicals
- Cuminum cyminum [2][3][4]
- Melaleuca alternifolia
- "cannabis sativa"
References
- ↑ Dewick, P. M. (2009). Medicinal Natural Products: A Biosynthetic Approach. United Kingdom: John Wiley & Sons. pp. 187–197.
- ↑ Li, Rong; Zi-Tao Jiang (2004). "Chemical composition of the essential oil of Cuminum cyminum L. from China". Flavour and Fragrance Journal 19 (4): 311–313. doi:10.1002/ffj.1302.
- ↑ Wang, Lu et al.; Wang, Z; Zhang, H; Li, X; Zhang, H (2009). "Ultrasonic nebulization extraction coupled with headspace single drop microextraction and gas chromatography–mass spectrometry for analysis of the essential oil in Cuminum cyminum L.". Analytica Chimica Acta 647 (1): 72–77. doi:10.1016/j.aca.2009.05.030. PMID 19576388.
- ↑ Iacobellis, Nicola S. et al.; Lo Cantore, P; Capasso, F; Senatore, F (2005). "Antibacterial Activity of Cuminum cyminum L. and Carum carvi L. Essential Oils". Journal of Agricultural and Food Chemistry 53 (1): 57–61. doi:10.1021/jf0487351. PMID 15631509.