Telomerization (dimerization)
The telomerization is the linear dimerization of 1,3-dienes with simultaneous addition of a nucleophile in a catalytic reaction.
Reaction
The reaction was independently discovered by E. J. Smutny at Shell and Takahashi at the Osaka University in the late sixties. The general reaction equation is as follows:[1][2]
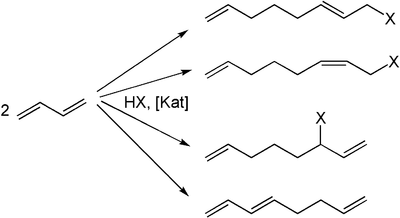
The formation of several isomers are possible. In addition to 1,3-butadiene also substituted dienes such as isoprene or cyclic dienes such as cyclopentadiene can be used. A variety of substances such as water, ammonia, alcohols, or C-H-acidic compounds can be used as nucleophiles. When water is used, for example di-unsaturated alcohols are obtained.
The catalysts used are mainly metal-organic palladium and nickel compounds. In 1991, Kuraray implemented the production of 1-octanol on an industrial scale (5000 t a(-1)).
The commercial route to produce 1-octene based on butadiene as developed by Dow Chemical came on stream in Tarragona in 2008. The telomerization of butadiene with methanol in the presence of a palladium catalyst yields 1-methoxy-2,7-octadiene, which is fully hydrogenated to 1-methoxyoctane in the next step. Subsequent cracking of 1-methoxyoctane gives 1-octene and methanol for recycle.
External links
Literature
- P. Fischer: process concepts for the transition-telomerization of isoprene with water or methanol. Shaker Verlag, 2002, 176 pages, ISBN 3-8322-0414-8, ISBN 978-3-8322-0414-3
- Arno Behr, Marc Becker, Thomas Beckmann, Leif Johnen, Julia Leschinski, Sebastian Reyer: Telomerization: Advances and Applications of a Versatile Reaction. In: Angewandte Chemie International Edition. 48, 2009, p. 3598–3614, doi:10.1002/anie.200804599.
- M.J.-L. Tschan, E.J. Garcıa-Suarez, Z. Freixa, H. Launay, H. Hagen, J. Benet-Buchholz, P.W.N.M. van Leeuwen, J. Am. Chem. Soc. 2010, 132, 6463-6473.
See also
References
- ↑ Edgar J. Smutny: Oligomerization and dimerization of butadiene under homogeneous catalysis. Reaction with nucleophiles and the synthesis of 1,3,7-octatriene In: Journal of the American Chemical Society. 89, 1967, p. 6793–6794, doi:10.1021/ja01001a089.
- ↑ S. Takahashi, T. Shibano, and N. Hagihara: The dimerization of butadiene by palladium complex catalysts. In: Tetrahedron Letters 8.26 (1967): 2451-2453.