Telmisartan
 | |
|---|---|
| Systematic (IUPAC) name | |
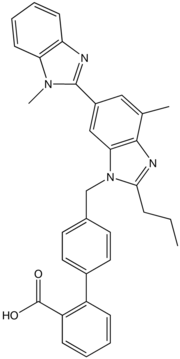
| 2-(4-{[4-methyl-6-(1-methyl-1H-1,3-benzodiazol-2-yl)-2-propyl-1H-1,3-benzodiazol-1-yl]methyl}phenyl)benzoic acid | |
| Clinical data | |
| Trade names | Micardis |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a601249 |
| Pregnancy cat. | D (Au), D (U.S.) |
| Legal status | S4 (Au), POM (UK), ℞-only (U.S.) |
| Routes | Oral |
| Pharmacokinetic data | |
| Bioavailability | 42–100% |
| Protein binding | ≥99.5% |
| Metabolism | Minimal hepatic |
| Half-life | 24 hours |
| Excretion | Faecal 97% |
| Identifiers | |
| CAS number | 144701-48-4 |
| ATC code | C09CA07 |
| PubChem | CID 65999 |
| IUPHAR ligand | 592 |
| DrugBank | DB00966 |
| ChemSpider | 59391 |
| UNII | U5SYW473RQ |
| KEGG | D00627 |
| ChEBI | CHEBI:9434 |
| ChEMBL | CHEMBL1017 |
| Chemical data | |
| Formula | C33H30N4O2 |
| Mol. mass | 514.617 g/mol |
| SMILES
| |
| |
| | |
Telmisartan (INN) /tɛlmɪˈsɑrtən/ is an angiotensin II receptor antagonist (angiotensin receptor blocker, ARB) used in the management of hypertension. It is marketed under the trade name Micardis (by Boehringer Ingelheim), among others.
Indication
Telmisartan is indicated in the treatment of essential hypertension.[1][2]
Administration
The usually effective dose telmisartan is 40–80 mg once daily. Some patients may already benefit at a daily dose of 20 mg. In cases where the target blood pressure is not achieved, telmisartan dose can be increased to a maximum of 80 mg once daily.[1]
Contraindications
Telmisartan is contraindicated during pregnancy. Like other drugs affecting the renin-angiotensin system (RAS), telmisartan can cause birth defects, stillbirths, and neonatal deaths. It is not known whether the drug passes into the breast milk.[3] Also it is contraindicated in bilateral renal artery stenosis in which it can cause renal failure.
Side effects
Side effects are similar to other angiotensin II receptor antagonists and include tachycardia and bradycardia (fast or slow heartbeat), hypotension (low blood pressure), edema (swelling of arms, legs, lips, tongue, or throat, the latter leading to breathing problems), and allergic reactions.[3]
Mode of action
Telmisartan is an angiotensin II receptor blocker that shows high affinity for the angiotensin II receptor type 1 (AT1), with a binding affinity 3000 times greater for AT1 than AT2. It has the longest half-life of any ARB (24 hours)[1][4] and the largest volume of distribution among ARBs (500 liters).[5][6]
In addition to blocking the RAs, telmisartan acts as a selective modulator of peroxisome proliferator-activated receptor gamma (PPAR-γ), a central regulator of insulin and glucose metabolism. It is believed that telmisartan’s dual mode of action may provide protective benefits against the vascular and renal damage caused by diabetes and cardiovascular disease (CVD).[4]
Telmisartan's activity at the PPAR-γ receptor has prompted speculation around its potential as a sport doping agent as an alternative to GW 501516.[7] Telmisartan activates PPARδ receptors in several tissues.[8][9][10][11]
Clinical trials
ONTARGET
The Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET) was one of the largest ARB clinical studies ever undertaken;[12] 25,620 patients from 733 centres in 41 countries were randomised for 5.5 years of treatment of either telmisartan, the ACE inhibitor ramipril or a combination of the two. The study aimed to investigate the role of telmisartan in cardiovascular (CV) protection through the primary composite outcome of death from CV causes, myocardial infarction, stroke or hospilization for heart failure, in high CV risk patients.
The study showed telmisartan was as effective as ramipril but with lower rates of cough and angioedema, which led to fewer discontinuations. The combination group experienced similar efficacy, but with increased risk of hypotensive symptoms. Moreover, in a patient population selected to tolerate ACE inhibitors, telmisartan was shown to be better tolerated and associated with higher treatment compliance than ramipril.[13]
TRANSCEND
As part of the ONTARGET study, patients who could not tolerate ACE inhibitors were randomly assigned to receive either telmisartan or placebo as part of the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (TRANSCEND) study. An accompanying editorial comments: "Overall, data supporting use of angiotensin-receptor blockers to prevent vascular events in various cardiovascular groups, other than heart failure, are incomplete. TRANSCEND's results challenge the non-inferiority shown in ONTARGET and suggest no more than a modest effect, if any at all."[14]
PRoFESS
The Prevention Regimen For Effectively Avoiding Second Strokes (PRoFESS) study investigated therapy with telmisartan initiated soon after an ischemic stroke and continued for 2.5 years. This treatment did not significantly lower the rate of recurrent stroke, major cardiovascular events, or diabetes.[15]
See also
References
- ↑ 1.0 1.1 1.2 Pritor prescribing information
- ↑ Drugs.com: Telmisartan
- ↑ 3.0 3.1 Drugs.com: Micardis
- ↑ 4.0 4.1 Benson, S. C.; Pershadsingh, H.; Ho, C.; Chittiboyina, A.; Desai, P.; Pravenec, M.; Qi, N.; Wang, J.; Avery, M.; Kurtz, T. W. (2004). "Identification of Telmisartan as a Unique Angiotensin II Receptor Antagonist with Selective PPAR -Modulating Activity". Hypertension 43 (5): 993–1002. doi:10.1161/01.HYP.0000123072.34629.57. PMID 15007034.
- ↑ Department of Pharmacokinetics and Drug Metabolism, Biberach an der Riss, Boehringer Ingelheim Pharma KG, Biberach, Germany (July 2000). "Pharmacokinetics of orally and intravenously administered telmisartan in healthy young and elderly volunteers and in hypertensive patients.". Journal of International Medical Research. Retrieved 18 January 2014.
- ↑ Philippe Gosse (September 2006). "A Review of Telmisartan in the Treatment of Hypertension: Blood Pressure Control in the Early Morning Hours". Vasc Health Risk Manag. Retrieved 18 January 2014.
- ↑ Sanchis-Gomar, F.; Lippi, G. (2011). "Telmisartan as metabolic modulator: A new perspective in sports doping?". Journal of Strength and Conditioning Research: 1. doi:10.1519/JSC.0b013e31824301b6. PMID 22130396.
- ↑ Cytoplasmic and Nuclear Receptors: Advances in Research and Application: 2011 Edition. ScholarlyEditions. 2012. pp. 21–. ISBN 978-1-464-93110-9. Retrieved 2 April 2013.
- ↑ Feng, X.; Luo, Z.; Ma, L.; Ma, S.; Yang, D.; Zhao, Z.; Yan, Z.; He, H.; Cao, T.; Liu, D.; Zhu, Z. (2011). "Angiotensin II receptor blocker telmisartan enhances running endurance of skeletal muscle through activation of the PPAR-δ/AMPK pathway". Journal of Cellular and Molecular Medicine 15 (7): 1572–1581. doi:10.1111/j.1582-4934.2010.01085.x. PMID 20477906.
- ↑ He, H.; Yang, D.; Ma, L.; Luo, Z.; Ma, S.; Feng, X.; Cao, T.; Yan, Z.; Liu, D.; Tepel, M.; Zhu, Z. (2010). "Telmisartan Prevents Weight Gain and Obesity Through Activation of Peroxisome Proliferator-Activated Receptor- -Dependent Pathways". Hypertension 55 (4): 869–879. doi:10.1161/HYPERTENSIONAHA.109.143958. PMID 20176998.
- ↑ Li, L.; Luo, Z.; Yu, H.; Feng, X.; Wang, P.; Chen, J.; Pu, Y.; Zhao, Y.; He, H.; Zhong, J.; Liu, D.; Zhu, Z. (2012). "Telmisartan Improves Insulin Resistance of Skeletal Muscle Through Peroxisome Proliferator-Activated Receptor- Activation". Diabetes 62 (3): 762–774. doi:10.2337/db12-0570. PMC 3581229. PMID 23238297.
- ↑ Ontarget, I.; Yusuf, S.; Teo, K.; Pogue, J.; Dyal, L.; Copland, I.; Schumacher, H.; Dagenais, G.; Sleight, P.; Anderson, C. (2008). "Telmisartan, Ramipril, or Both in Patients at High Risk for Vascular Events". New England Journal of Medicine 358 (15): 1547–1559. doi:10.1056/NEJMoa0801317. PMID 18378520.
- ↑ Bayer Healthcare: Telmisartan approved by the European Commission to reduce the risk of cardiovascular (CV) morbidity in a broad spectrum of at risk patients
- ↑ Ripley, T. L.; Harrison, D. (2008). "The power to TRANSCEND". The Lancet 372 (9644): 1128. doi:10.1016/S0140-6736(08)61243-X. PMID 18757086.
- ↑ ClinicalTrials.gov NCT00153062 PRoFESS - Prevention Regimen For Effectively Avoiding Second Strokes
| ||||||||||||||||||||