Tantalum(IV) sulfide
| Tantalum(IV) sulfide | ||
|---|---|---|
 | ||
| Other names tantalum disulfide | ||
| Identifiers | ||
| CAS number | 12143-72-5 | |
| PubChem | 82945 | |
| Jmol-3D images | {{#if:S=[Ta]=S|Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | TaS2 | |
| Molar mass | 245.08 g/mol | |
| Appearance | black crystals | |
| Density | 6.86 g/cm³, solid | |
| Melting point | 3000°C | |
| Structure | ||
| Crystal structure | hexagonal | |
| Hazards | ||
| EU classification | not listed | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Tantalum(IV) sulfide is the inorganic compound with the formula TaS2. It is a layered compound with three-coordinate sulfide centres and trigonal prismatic metal centres. It is structurally similar to the more famous material molybdenum disulfide, MoS2. TaS2 is a semiconductor with d1 electron configuration.
Although an obscure material otherwise, TaS2 has been the subject of many studies because it is a versatile host for intercalation of electron donors.[1]
TaS2 is prepared by the high temperature reaction of powdered tantalum and sulfur. It is purified and crystallized by chemical vapor transport using iodine as the transporting agent:
- TaS2 + 2 I2
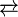 TaI4 + 2 S
TaI4 + 2 S
The crystallographic structure of tantalum disulfide shows a number of complexities. One of its forms 1T-TaS2 undergoes a number of phase transitions as a function of temperature. In the lowest temperature phase, the commensurate phase (below 180K) there is a commensurate superstructure, known as a charge density wave (CDW), at an angle of 13.9 degrees relative to the lattice. In an incommensurate phase above 353K the wave is aligned with the lattice. Two more structures exist: a nearly commensurate one appears between 353K and 180K upon cooling. Upon warming a triclinic phase is first formed between 223K and 283K to revert to the nearly commensurate one at 283K. Both these phases have different superstructures.[2]
References
- ↑ J. F. Revelli, "Tantalum Disulfide (TaS2) and Its Intercalation Compounds" Inorganic Syntheses 1995, volume 30, pp. 155. doi:10.1002/9780470132616.ch32
- ↑ Scanning Tunneling Microscopy of Charge Density Wave Structure in Tantalum Disulfide Thomson, Ruth Ellen Thesis (PH.D.)--UNIVERSITY OF CALIFORNIA, BERKELEY, 1991.Source: Dissertation Abstracts International, Volume: 53-05, Section: B, page: 2385.
| |||||