Tandamine
From Wikipedia, the free encyclopedia
 | |
|---|---|
| Systematic (IUPAC) name | |
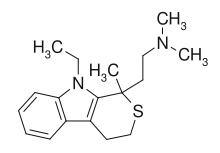
| 2-(9-ethyl-1-methyl-3,4-dihydrothiopyrano[3,4-b]indol-1-yl)-N,N-dimethylethanamine | |
| Clinical data | |
| Legal status | ? |
| Identifiers | |
| CAS number | 42408-80-0 |
| ATC code | None |
| PubChem | CID 39187 |
| UNII | 7516K9UGUG |
| Chemical data | |
| Formula | C18H26N2S |
| Mol. mass | 302.476 g/mol |
| SMILES
| |
| | |
Tandamine is a selective norepinephrine reuptake inhibitor with a tricyclic structure.[1][2][3] It was developed in the 1970s as an antidepressant but was never commercialized.[1][2][3] Tandamine is analogous to pirandamine, which, instead, acts as a selective serotonin reuptake inhibitor (SSRI).[4][5]
See also
References
- ↑ 1.0 1.1 Lippmann W, Pugsley TA (May 1976). "The effects of tandamine, a new potential antidepressant agent, on biogenic amine uptake mechanisms and related activities". Biochemical Pharmacology 25 (10): 1179–86. doi:10.1016/0006-2952(76)90366-X. PMID 1084746.
- ↑ 2.0 2.1 Ehsanullah RS, Ghose K, Kirby MJ, Turner P, Witts D (March 1977). "Clinical pharmacological studies of tandamine, a potential antidepressive drug". Psychopharmacology 52 (1): 73–7. doi:10.1007/BF00426603. PMID 403562.
- ↑ 3.0 3.1 Pugsley TA, Lippmann W (September 1979). "Effect of acute and chronic treatment of tandamine, a new heterocyclic antidepressant, on biogenic amine metabolism and related activities". Naunyn-Schmiedeberg's Archives of Pharmacology 308 (3): 239–47. doi:10.1007/BF00501388. PMID 503251.
- ↑ Pugsley T, Lippmann W (May 1976). "Effects of tandamine and pirandamine, new potential antidepressants, on the brain uptake of norepinephrine and 5-hydroxytryptamine and related activities". Psychopharmacology 47 (1): 33–41. doi:10.1007/BF00428698. PMID 1085452.
- ↑ Lippmann W, Seethaler K (April 1977). "Effects of tandamine and pirandamine, selective blockers of biogenic amine uptake mechanisms, on gastric acid secretion and ulcer formation in the rat". Life Sciences 20 (8): 1393–400. doi:10.1016/0024-3205(77)90367-8. PMID 853871.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.