TCEP
| TCEP | |
|---|---|
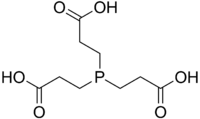 | |
| IUPAC name 3,3′,3′′-Phosphanetriyltripropanoic acid | |
| Other names TCEP | |
| Identifiers | |
| CAS number | 5961-85-3 |
| PubChem | 119411 |
| ChemSpider | 106653 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C9H15O6P |
| Molar mass | 250.19 g mol−1 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
TCEP (tris(2-carboxyethyl)phosphine) is a reducing agent frequently used in biochemistry and molecular biology applications.[1] It is often prepared and used as a hydrochloride salt (TCEP-HCl) with a molecular weight of 286.65 gram/mol. It is soluble in water and available as a stabilized solution at neutral pH and immobilized onto an agarose support to facilitate removal of the reducing agent.
Applications
TCEP is often used as a reducing agent to break disulfide bonds within and between proteins as a preparatory step for gel electrophoresis.
Compared to the other two most common agents used for this purpose (dithiothreitol and β-mercaptoethanol), TCEP has the advantages of being odorless, a more powerful reducing agent, an irreversible reducing agent (in the sense that TCEP does not regenerate--the end product of TCEP-mediated disulfide cleavage is in fact two free thiols/cysteines), more hydrophilic, and more resistant to oxidation in air.[2] It also does not reduce metals used in immobilized metal affinity chromatography.
TCEP is particularly useful when labeling cysteine residues with maleimides. TCEP can keep the cysteines from forming di-sulfide bonds and unlike dithiothreitol and β-mercaptoethanol, it will not react as readily with the maleimide.[2] However, TCEP has been reported to react with maleimide under certain conditions.[3][4]
TCEP is also used in the tissue homogenization process for RNA isolation.
References
- ↑ Ruegg, U.T and Rudinger, J. (1977). "Reductive cleavage of cystine disulfides with tributylphosphine". Methods Enzymol. Methods in Enzymology 47: 111–116. doi:10.1016/0076-6879(77)47012-5. ISBN 978-0-12-181947-7. PMID 927167.
- ↑ 2.0 2.1 TCEP technical information, from Interchim
- ↑ Shafer, D. E.; Inman, J. K.; Lees, A. (2002). "Reaction of Tris(2-carboxyethyl)phosphine (TCEP) with Maleimide and α-Haloacyl Groups: Anomalous Elution of TCEP by Gel Filtration". Anal. Biochem. 282 (1): 161–164. doi:10.1006/abio.2000.4609. PMID 10860517.
- ↑ Tyagarajan K, Pretzer E, Wiktorowicz JE (2003). "Thiol-reactive dyes for fluorescence labeling of proteomic samples". Electrophoresis 24 (14): 2348–2358. doi:10.1002/elps.200305478. PMID 12874870.