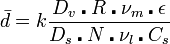Suspension polymerization
Suspension polymerization is a heterogeneous radical polymerization process that uses mechanical agitation to mix a monomer or mixture of monomers in a liquid phase, such as water, while the monomers polymerize, forming spheres of polymer.
This process is used in the production of many commercial resins, including polyvinyl chloride (PVC), a widely used plastic, styrene resins including polystyrene, expanded polystyrene, and high-impact polystyrene, as well as poly(styrene-acrylonitrile) and poly(methyl methacrylate).[1]
Polymerization in which polymer is formed in monomer, or monomer-solvent droplets
in a continuous phase that is a nonsolvent for both the monomer and the formed polymer.
Note 1: In suspension polymerization, the initiator is located mainly in the monomer phase.
Note 2: Monomer or monomer-solvent droplets in suspension polymerization have
diameters usually exceeding 10 μm.[2]
Reaction conditions
The reaction mixture consists of two phases, a liquid matrix and monomer droplets. The monomer and initiator are insoluble in the liquid phase, so they form drops within the liquid matrix. A suspension agent is usually added to stabilize the monomer droplets and hinder monomer drops from coming together. The reaction mixture usually has a volume ratio of monomer to liquid phase of 0.1 to 0.5. The liquid phase acts as a heat transfer agent, enabling high rates of polymerization with little change in the temperature of the polymerizing solution. The reactions are usually done in a stirred tank reactor that continuously mixes the solution using turbulent pressure or viscous shear forces. The stirring action helps to keep the monomer droplets separated and creates a more uniform suspension, which leads to a more narrow size distribution of the final polymer beads. The polymerization is usually carried to completion.[3] The kinetics of the polymerization within an individual bead are similar to those of typical radical polymerization.[4]
Particle properties
Suspension polymerization is divided into two main types, depending on the morphology of the particles that result. In bead polymerization, the polymer is soluble in its monomer and the result is a smooth, translucent bead. In powder polymerization, the polymer is not soluble in its monomer and the resultant bead will be porus and irregular.[5] The morphology of the polymer can be changed by adding a monomer diluent, an inert liquid that is insoluble with the liquid matrix. The diluent changes the solubility of the polymer in the monomer and gives a measure of control over the porosity of the resulting polymer.[3]
The polymer beads that result can range in size from 100 nm to 5 mm. The size is controlled by the stirring speed, the volume fraction of monomer, the concentration and identity of the stabilizers used, and the viscosities of the different components. The following equation derived empirically summarizes some of these interactions:
d is the average particle size, k includes parameters related to the reaction vessel design, Dv is the reaction vessel diameter, Ds is the diameter of the stirrer, R is the volume ratio of the monomer to the liquid matrix, N is the stirring speed, νm and νl are the viscosity of the monomer phase and liquid matrix respectively, ε is the interfacial tension of the two phases, and Cs is the concentration of stabilizer. The most common way to control the particle size is to change the stirring speed.[3]
See also
References
- ↑ Vivaldo-Lima, E., Wood, P., and Hamielec, A. (1997). "An Updated Review on Suspension Polymerization". Ind. Eng. Chem. Res. 36: 939–965. doi:10.1021/ie960361g.
- ↑ "Terminology of polymers and polymerization processes in dispersed systems (IUPAC Recommendations 2011)". Pure and Applied Chemistry 83 (12): 2229–2259. 2011. doi:10.1351/PAC-REC-10-06-03.
- ↑ 3.0 3.1 3.2 Arshady, R. (1992). "Suspension, Emulsion, and Dispersion Polymerization: A Methodological Survey". Colloid. Polym. Sci. 270: 717–732. doi:10.1007/BF00776142.
- ↑ Kalfas, G., Yuan, H., and Ray, W. (1993). "Modeling and Experimental Studies of Aqueous Suspension Polymerization Processes. 2. Experiments in Batch Reactors". Ind. Eng. Chem. Res. 32: 1831–1838. doi:10.1021/ie00021a006.
- ↑ Kotoulas, Costas, and Kiparissides, Costas (2006). "A Generalized Population Balance Model for the Prediction of Particle Size Distribution in Suspension Polymerization Reactors". Chemical Engineering Science 61: 332–346. doi:10.1016/j.ces.2005.07.013.