Susceptibility weighted imaging
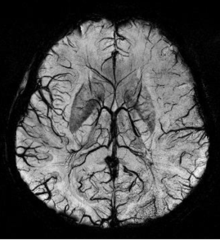
Susceptibility weighted imaging (SWI), originally called BOLD venographic imaging, uses a type of contrast in magnetic resonance imaging (MRI) different from traditional spin density, T1, or T2 imaging. SWI uses a fully flow compensated, long echo, gradient recalled echo (GRE) pulse sequence to acquire images. This method exploits the susceptibility differences between tissues and uses the phase image to detect these differences. The magnitude and phase data are combined to produce an enhanced contrast magnitude image which is exquisitely sensitive to venous blood, hemorrhage and iron storage. The imaging of venous blood with SWI is a blood-oxygen-level dependent (BOLD) technique which is why it was (and is sometimes still) referred to as BOLD venography. Due to its sensitivity to venous blood SWI is commonly used in traumatic brain injuries (TBI) and for high resolution brain venographies but has many other clinical applications. SWI is offered as a clinical package by Philips and Siemens but can be run on any manufacturer’s machine at field strengths of 1.0 T, 1.5 T, 3.0 T and higher.
Acquisition and image processing
SWI uses a fully velocity compensated, three dimensional, RF spoiled, high-resolution, 3D gradient recalled echo (GRE)scan. Both the magnitude and phase images are saved, and the phase image is high pass (HP) filtered to removed unwanted artifacts. The magnitude image is then combined with the phase image to create an enhanced contrast magnitude image referred to as the susceptibility weighted (SW) image. It is also common to create minimum intensity projections (mIP) over 8 to 10 mm to better visualize vein connectivity. In this way four sets of images are generated, the original magnitude, HP filtered phase, susceptibility weighted, and mIPs over the susceptibility weighted images.
Phase filtering
The values in the phase images are constrained from -π to π so if the value goes above π it wraps to -π. Inhomogeneities in the magnetic field cause low frequency background gradients. This causes all the phase values to slowly increase across the image which creates phase wrapping and obscures the image. This type of artifact can be removed by phase unwrapping or by high pass filtering the original complex data to remove the low frequency variations in the phase image.
Susceptibility weighted image creation

The susceptibility weighted image is created by combining the magnitude and filtered phase images. A mask is created from the phase image by mapping all values above 0 radians to be 1 and linearly mapping values from -π to 0 radians to range from 0 to 1, respectively. Alternatively, a power function (typically 4th degree) can be used instead of a linear mapping from -π to 0 to increase the effect of the mask. The magnitude image is then multiplied by this mask. In this way phase values above 0 radians have no effect and phase values below 0 radians darken the magnitude image. This increases the contrast in the magnitude image for objects with low phase values such as veins, iron, and hemorrhage.
Clinical applications
Clinical applications are under research in different fields of medicine.[1] [2]
Traumatic brain injury (TBI)


The detection of micro-hemorrhages, shearing, and diffuse axonal injury (DAI) in trauma patients is often difficult as the injuries tend to be relatively small in size and can be easily missed by low resolution scans. SWI is usually run at relatively high resolution (1 mm3) and is extremely sensitive to bleeding in the gray matter/white matter boundaries making it is possible to see very small lesions increasing the ability to detect more subtle injuries.
Stroke and hemorrhage
Diffusion weighted imaging offers a powerful means to detect acute stroke. Although it is well known that gradient echo imaging can detect hemorrhage, it is best detected with SWI. In the example shown here, the gradient echo image shows the region of likely cytotoxic edema whereas the SW image shows the likely localization of the stroke and the vascular territory affected (data acquired at 1.5 T).
The bright region in the gradient echo weighted image shows the area affected in this acute stroke example. The arrows in the SWI image may show the tissue at risk that has been affected by the stroke (A, B, C) and the location of the stroke itself (D). The reason that we are able to see the affected vascular territory could be because there is a reduced level of oxygen saturation in this tissue, suggesting that the flow to this region of the brain could be reduced post stroke. Another possible explanation is that there is an increase in local venous blood volume. In either case, this image suggests that the tissue associated with this vascular territory could be tissue at risk. Future stroke research will involve comparisons of perfusion weighted imaging and SWI to learn more about local flow and oxygen saturation.
Sturge-Weber disease

An SWI venogram of a neonate with Sturge-Weber syndrome who did not display neurological symptoms is shown to the left. The initial conventional MR imaging methods did not demonstrate any abnormality. The abnormal venous vasculature in the left occipital lobe extending between the posterior horn of the ventricle and the cortical surface is clearly visible in the venogram. Due to the high resolution even collaterals can be resolved.
Tumors
Part of the characterization of tumors lies in understanding the angiographic behavior of lesions both from the perspective of angiogenesis and micro-hemorrhages. Aggressive tumors tend to have rapidly growing vasculature and many micro-hemorrhages. Hence, the ability to detect these changes in the tumor could lead to a better determination of the tumor status. The enhanced sensitivity of SWI to venous blood and blood products due to their differences in susceptibility compared to normal tissue leads to better contrast in detecting tumor boundaries and tumor hemorrhage.
Multiple sclerosis
Multiple sclerosis (MS) is usually studied with FLAIR and contrast enhanced T1 imaging. SWI adds to this by revealing the venous connectivity in some lesions and presents evidence of iron in some lesions. This key new information may help understand the physiology of MS.[3]
The phase images acquired with an SWI scan were shown to be sensitive to MS lesion formation.[4]
Vascular dementia and cerebral amyloid angiopathy (CAA)

Gradient recalled echo (GRE) imaging is the conventional way to detect hemorrhage in CAA, however SWI is a much more sensitive technique that can reveal many micro-hemorrhages that are missed on GRE images.[5] A conventional gradient echo T2*-weighted image (left, TE=20 ms) shows some low-signal foci associated with CAA. On the other hand, an SWI image (center, with a resolution of 0.5 mm x 0.5 mm x 2.0 mm, projected over 8mm) shows many more associated low-signal foci. Phase images were used to enhance the effect of the local hemosiderin build-up. An example phase image (right) with yet higher resolution of 0.25 mm x 0.25 mm x 2.0 mm shows a clear ability to localize multiple CAA-associated foci.
Pneumocephalus
Recent studies suggest that SWI might be suitable for monitorizing neurosurgical patients recovering from Pneumocephalus, as air can be easily detected with SWI.
High field SWI
SWI is uniquely suited to take advantage of higher field systems, as the contrast in the phase image is linearly proportional to echo time (TE) and field strength. Higher fields thus allow shorter echo times without a loss of contrast which can reduce scan time and motion related artifacts. The high signal-to-noise available at higher fields also increases scan quality and allows for higher resolution scans.[6]
See also
External links
- SWI information brochures, including SWI software
- MRI-CCSVI Pilot Study with MRA and SWI
- SWI-MRI research center
- NICE MRI
- MRI institute for biomedical research
Footnotes
- ↑ Mittal S, Wu Z, Neelavalli J, Haacke EM (February 2009). "Susceptibility-weighted imaging: technical aspects and clinical applications, part 2". AJNR Am J Neuroradiol 30 (2): 232–52. doi:10.3174/ajnr.A1461. PMID 19131406.
- ↑ Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC (January 2009). "Susceptibility-weighted imaging: technical aspects and clinical applications, part 1". AJNR Am J Neuroradiol 30 (1): 19–30. doi:10.3174/ajnr.A1400. PMID 19039041.
- ↑ Haacke EM, Makki M, Ge Y, Maheshwari M, Sehgal V, Hu J, Selvan M, Wu Z, Latif Z, Xuan Y, Khan O, Garbern J, Grossman RI (March 2009). "Characterizing iron deposition in multiple sclerosis lesions using susceptibility weighted imaging". J Magn Reson Imaging 29 (3): 537–44. doi:10.1002/jmri.21676. PMC 2650739. PMID 19243035.
- ↑ Wiggermann V, Hernandez Torres E, Vavsour IM, Moore GR, Laule C, MacKay AL, Li DK Z, Traboulsee A, Rauscher A (July 2013). "Magnetic resonance frequency shifts during acute MS lesion formation.". Neurology. PMID 23761621.
- ↑ Haacke EM et al. (2007). "Imaging Cerebral Amyloid Angiopathy with Susceptibility-Weighted Imaging". American Journal of Neuroradiology 28 (2): 316–7. PMID 17297004.
- ↑ Deistung A et al. (2008). "Susceptibility weighted imaging at ultra high magnetic field strengths: theoretical considerations and experimental results". Magn Reson Med 60 (5): 1155–68. doi:10.1002/mrm.21754. PMID 18956467..
References
- Ashwal S et al. (2008). "Susceptibility-weighted imaging and proton magnetic resonance spectroscopy in assessment of outcome after pediatric traumatic brain injury". Arch Phys Med Rehabil 87 (12 Suppl 2): S50–8. doi:10.1016/j.apmr.2006.07.275. PMID 17140880.
- Barth M et al. (2003). "High-resolution three-dimensional contrast-enhanced blood oxygenation level-dependent magnetic resonance venography of brain tumors at 3 Tesla: first clinical experience and comparison with 1.5 Tesla". Invest Radiol 38 (7): 409–14. doi:10.1097/01.RLI.0000069790.89435.e7. PMID 12821854.
- Deistung A et al. (2008). "Susceptibility weighted imaging at ultra high magnetic field strengths: theoretical considerations and experimental results". Magn Reson Med 60 (5): 1155–68. doi:10.1002/mrm.21754. PMID 18956467.
- Denk and Rauscher; Rauscher, A (2010). "Susceptibility weighted imaging with multiple echoes". Journal of Magnetic Resonance Imaging 31 (1): 185–91. doi:10.1002/jmri.21995. PMID 20027586.
- de Souza JM et al. (2008). "Susceptibility-weighted imaging for the evaluation of patients with familial cerebral cavernous malformations: a comparison with t2-weighted fast spin-echo and gradient-echo sequences". Am J Neuroradiol 29 (1): 154–8. doi:10.3174/ajnr.A0748. PMID 17947370.
- Haacke EM et al. (2005). "Imaging iron stores in the brain using magnetic resonance imaging". Magn Reson Imaging 23 (1): 1–25. doi:10.1016/j.mri.2004.10.001. PMID 15733784.
- Haacke EM et al. (2009). "Susceptibility-weighted imaging: technical aspects and clinical applications, part 1". Am J Neuroradiol 30 (1): 19–30. doi:10.3174/ajnr.A1400. PMID 19039041.
- Mittal S et al. (2009). "Susceptibility-Weighted Imaging: Technical Aspects and Clinical Applications, Part 2". Am J Neuroradiol 30 (2): 232–52. doi:10.3174/ajnr.A1461. PMID 19131406.
- Rauscher A et al. (April 1, 2005). "Magnetic susceptibility-weighted MR phase imaging of the human brain". Am J Neuroradiol 26 (4): 736–42. PMID 15814914.
- Reichenbach JR et al. (July 1, 1997). "Small vessels in the human brain: MR venography with deoxyhemoglobin as an intrinsic contrast agent". Radiology 204 (1): 272–7. PMID 9205259.
- Reichenbach JR et al. (2000). "High-resolution MR venography at 3.0 Tesla". J Comput Assist Tomogr 24 (6): 949–57. doi:10.1097/00004728-200011000-00023. PMID 11105717.
- Reichenbach JR et al. (2001). "High-resolution BOLD venographic imaging: a window into brain function". NMR Biomed 14 (7–8): 453–67. doi:10.1002/nbm.722. PMID 11746938.
- Sedlacik J et al. (2007). "Obtaining blood oxygenation levels from MR signal behavior in the presence of single venous vessels". Magn Reson Med 58 (5): 1035–44. doi:10.1002/mrm.21283. PMID 17969121.
- Sehgal V et al. (2005). "Clinical applications of neuroimaging with susceptibility-weighted imaging". J Magn Reson Imaging 22 (4): 439–50. doi:10.1002/jmri.20404. PMID 16163700.
- Sehgal V et al. (2006). "Susceptibility-weighted imaging to visualize blood products and improve tumor contrast in the study of brain masses". J Magn Reson Imaging 24 (1): 41–51. doi:10.1002/jmri.20598. PMID 16755540.
- Thomas B et al. (2008). "Clinical applications of susceptibility weighted MR imaging of the brain - a pictorial review". Neuroradiology 50 (2): 105–16. doi:10.1007/s00234-007-0316-z. PMID 17929005.
- Tong K et al. (2008). "Susceptibility-weighted MR imaging: a review of clinical applications in children". Am J Neuroradiol 29 (1): 9–17. doi:10.3174/ajnr.A0786. PMID 17925363.
- Witoszynskyj S et al. (2008). "Phase unwrapping of MR images using PhiUN - A fast and robust region growing algorithm". Med Image Anal 13 (2): 257–68. doi:10.1016/j.media.2008.10.004. PMID 19070532.
- Palma JA et al. (2009). "Pneumocephalus mimicking cerebral cavernous malformations in MR susceptibility-weighted imaging". AJNR Am J Neuroradiol 30 (6): e83. doi:10.3174/ajnr.A1549. PMID 19342538.