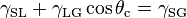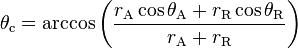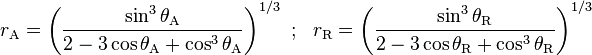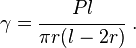Surface energy
Surface energy quantifies the disruption of intermolecular bonds that occur when a surface is created. In the physics of solids, surfaces must be intrinsically less energetically favorable than the bulk of a material (the molecules on the surface have more energy compared with the molecules in the bulk of the material), otherwise there would be a driving force for surfaces to be created, removing the bulk of the material (see sublimation). The surface energy may therefore be defined as the excess energy at the surface of a material compared to the bulk.
For a liquid, the surface tension (force per unit length) and the surface energy density are identical. Water has a surface energy density of 0.072 J/m2 and a surface tension of 0.072 N/m; the units are equivalent.
Cutting a solid body into pieces disrupts its bonds, and therefore consumes energy. If the cutting is done reversibly (see reversible), then conservation of energy means that the energy consumed by the cutting process will be equal to the energy inherent in the two new surfaces created. The unit surface energy of a material would therefore be half of its energy of cohesion, all other things being equal; in practice, this is true only for a surface freshly prepared in vacuum. Surfaces often change their form away from the simple "cleaved bond" model just implied above. They are found to be highly dynamic regions, which readily rearrange or react, so that energy is often reduced by such processes as passivation or adsorption.
Measuring the surface energy of a liquid
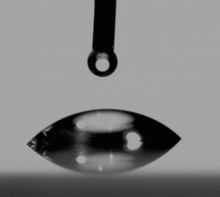
As first described by Thomas Young in 1805 in the Philosophical Transactions of the Royal Society of London, it is the interaction between the forces of cohesion and the forces of adhesion which determines whether or not wetting, the spreading of a liquid over a surface, occurs. If complete wetting does not occur, then a bead of liquid will form, with a contact angle which is a function of the surface energies of the system.

Surface energy is most commonly quantified using a contact angle goniometer and a number of different methods.

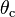 is the angle formed by a liquid at the three-phase boundary where the liquid, gas, and solid meet.
is the angle formed by a liquid at the three-phase boundary where the liquid, gas, and solid meet.Young's equation
Young established the well-regarded Young's Equation which defines the balances of forces caused by a wet drop on a dry surface. If the surface is hydrophobic then the contact angle of a drop of water will be larger. Hydrophilicity is indicated by smaller contact angles and higher surface energy. (Water has rather high surface energy by nature; it is polar and forms hydrogen bonds). The Young equation gives the following relation,
where 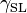 ,
,  , and
, and 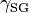 are the interfacial tensions between the solid and the liquid, the liquid and the vapor, and the solid and the vapor, respectively. The equilibrium contact angle that the drop makes with the surface is denoted by
are the interfacial tensions between the solid and the liquid, the liquid and the vapor, and the solid and the vapor, respectively. The equilibrium contact angle that the drop makes with the surface is denoted by 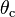 . To derive the Young equation, the interfacial tensions are described as forces per unit length and then an one-dimensional force equilibrium is established along the solid boundary.
. To derive the Young equation, the interfacial tensions are described as forces per unit length and then an one-dimensional force equilibrium is established along the solid boundary.
The Young equation assumes a perfectly flat surface, and in many cases surface roughness and impurities cause a deviation in the equilibrium contact angle from the contact angle predicted by Young's equation. Even in a perfectly smooth surface a drop will assume a wide spectrum of contact angles ranging from the so-called advancing contact angle, 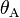 , to the so-called receding contact angle,
, to the so-called receding contact angle,  . The equilibrium contact angle (
. The equilibrium contact angle (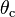 ) can be calculated from
) can be calculated from 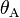 and
and  as was shown theoretically by Tadmor [1] and confirmed experimentally by Chibowski [2] as,
as was shown theoretically by Tadmor [1] and confirmed experimentally by Chibowski [2] as,
where
In the case of "dry wetting", one can use the Young-Dupré equation which is expressed by the work of adhesion. This method accounts for the surface pressure of the liquid's vapor which can be significant. Pierre-Gilles de Gennes, a Nobel Prize Laureate in Physics, describes wet and dry wetting and how the difference between the two relate to whether or not the vapor is saturated.[3]
Measuring the surface energy of a solid
The surface energy of a liquid may be measured by stretching a liquid membrane (which increases the surface area and hence the surface energy density). In that case, in order to increase the surface area of a mass of liquid by an amount, δA, a quantity of work, γδA, is needed (where γ is the surface energy density of the liquid). However, such a method cannot be used to measure the surface energy of a solid because stretching of a solid membrane induces elastic energy in the bulk in addition to increasing the surface energy.
The surface energy of a solid is usually measured at high temperatures. At such temperatures the solid creeps and even though the surface area changes, the volume remains approximately constant. If γ is the surface energy density of a cylindrical rod of radius  and length
and length  at high temperature and a constant uniaxial tension
at high temperature and a constant uniaxial tension  , then at equilibrium, the variation of the total Gibbs free energy vanishes and we have
, then at equilibrium, the variation of the total Gibbs free energy vanishes and we have
where  is the Gibbs free energy and
is the Gibbs free energy and 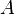 is the surface area of the rod:
is the surface area of the rod:
Also, since the volume ( ) of the rod remains constant, the variation (
) of the rod remains constant, the variation ( ) of the volume is zero, i.e.,
) of the volume is zero, i.e.,
Therefore, the surface energy density can be expressed as
The surface energy density of the solid can be computed by measuring  ,
,  , and
, and  at equilibrium.
at equilibrium.
This method is valid only if the solid is isotropic, meaning the surface energy is the same for all crystallographic orientations. While this is only strictly true for amorphous solids (glass) and liquids, isotropy is a good approximation for many other materials. In particular, if the sample is polygranular (most metals) or made by powder sintering (most ceramics) this is a good approximation.
In the case of single-crystal materials, such as natural gemstones, anisotropy in the surface energy leads to faceting. The shape of the crystal (assuming equilibrium growth conditions) is related to the surface energy by the Wulff construction. The surface energy of the facets can thus be found to within a scaling constant by measuring the relative sizes of the facets.
Calculating the surface energy of a deformed solid
In the deformation of solids, surface energy can be treated as the "energy required to create one unit of surface area", and is a function of the difference between the total energies of the system before and after the deformation:
 .
.
Calculation of surface energy from first principles is an alternative approach to measurement. Surface energy is estimated from the following variables: width of the d-band, the number of valence d-electrons, and the coordination number of atoms at the surface and in the bulk of the solid.[4]
Calculating the surface formation energy of a crystalline solid
In the ab initio calculations, formation energy of the crystalline solid, such as titanium (IV) oxide or magnesium oxide, can be obtained from the following equation:
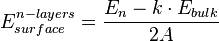
where  corresponds to the energy of the thin film of crystalline oxide, calculated from first principles, n stands for a number of atomic layers forming a model of the surface, while k is the number of repetitive units in a direction normal to the surface. A is the area of the primitive surface unit cell and the
corresponds to the energy of the thin film of crystalline oxide, calculated from first principles, n stands for a number of atomic layers forming a model of the surface, while k is the number of repetitive units in a direction normal to the surface. A is the area of the primitive surface unit cell and the 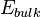 is the energy per atomic layer in three-dimensional system.[5]
is the energy per atomic layer in three-dimensional system.[5]
See also
- Contact angle
- Surface tension
- Sessile drop technique
- Capillary surface
- Wulff Construction
References
- ↑ Tadmor, Rafael (2004). "Line energy and the relation between advancing, receding and Young contact angles". Langmuir 20 (18): 7659. doi:10.1021/la049410h. PMID 15323516.
- ↑ Chibowski, Emil (2008). "Surface free energy of sulfur—Revisited I. Yellow and orange samples solidified against glass surface". Journal of Colloid and Interface Science 319: 505. doi:10.1016/j.jcis.2007.10.059.
- ↑ Pierre-Gilles de Gennes, Françoise Brochard-Wyart, David Quéré (2002). Capillary and Wetting Phenomena - Drops, Bubbles, Pearls, Waves. Alex Reisinger. Springer. ISBN 0-387-00592-7.
- ↑ D.P. Woodruff, ed. "The Chemical Physics of Solid Surfaces", Vol. 10, Elsevier, 2002.
- ↑ Bardziński, Piotr J. (2011). "Determination of the electronic band structure of the rutile polymorph of TiO2: a quantum chemical approach". Materials Science-Poland 29 (3): 227–228. Bibcode:2011MatSP..29..223B. doi:10.2478/s13536-011-0035-3.
| ||||||||||||||