Suramin
 | |
|---|---|
 | |
| Systematic (IUPAC) name | |
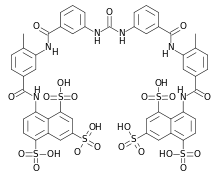
| 8,8'-{Carbonylbis[imino-3,1-phenylenecarbonylimino(4-methyl-3,1-phenylene)carbonylimino]}di(1,3,5-naphthalenetrisulfonic acid) | |
| Clinical data | |
| Trade names | Antrypol |
| Legal status | ? |
| Identifiers | |
| CAS number | 145-63-1 |
| ATC code | P01CX02 QP51AE02 |
| PubChem | CID 5361 |
| IUPHAR ligand | 1728 |
| DrugBank | DB04786 |
| ChemSpider | 5168 |
| UNII | 6032D45BEM |
| KEGG | C07974 |
| ChEBI | CHEBI:45906 |
| ChEMBL | CHEMBL265502 |
| Chemical data | |
| Formula | C51H40N6O23S6 |
| Mol. mass | 1297.29 |
| SMILES
| |
| |
| | |
Suramin is a drug developed by Oskar Dressel and Richard Kothe of Bayer, Germany in 1916, and is still sold by Bayer under the brand name Germanin.
According to the National Cancer Institute there are no active clinical trials (as of April 1, 2008). Completed and closed clinical trials are listed here:
In addition to Germanin, the National Cancer Institute also lists the following "Foreign brand names": 309 F or 309 Fourneau,[1] Bayer 205, Moranyl, Naganin, Naganine.
Uses
Protozoa
It is used for treatment of human sleeping sickness caused by trypanosomes.[2]
Helminthiasis
It has been used in the treatment of onchocerciasis.[3]
Other
It has been investigated as treatment for prostate cancer.[4]
Also, suramin as treatment for autism is being evaluated. [5]
Chemistry
The molecular formula of suramin is C51H34N6O23S6. It is a symmetric molecule in the center of which lies urea, NH-CO-NH. Suramin contains eight benzene rings, four of which are fused in pairs (naphthalene), four amide groups in addition to the one of urea and six sulfonate groups. When given as drug it usually contains six sodium ions that form a salt with the six sulfonate groups.
Dosing
Suramin is administered by a single weekly intravenous injection for six weeks. The dose per injection is 1 g.
Adverse reactions
The most frequent adverse reactions are nausea and vomiting. About 90% of patients will get an urticarial rash that disappears in a few days without needing to stop treatment. There is a greater than 50% chance of adrenal cortical damage, but only a smaller proportion will require lifelong corticosteroid replacement. It is common for patients to get a tingling or crawling sensation of the skin with suramin. Suramin will cause clouding of the urine which is harmless: patients should be warned of this to avoid them becoming alarmed.
Kidney damage and exfoliative dermatitis occur less commonly.
Suramin has been applied clinically to HIV/AIDS patients resulting in a significant number of fatal occurrences and as a result the application of this molecule was abandoned for this condition. http://www.ncbi.nlm.nih.gov/pubmed/3548350
Research
Suramin is also used in research as a broad-spectrum antagonist of P2 receptors[6][7] and agonist of Ryanodine receptors.[8]
Its effect on telomerase has been investigated.[9]
It may have some activity against RNA viruses.[10]
In addition to antagonism of P2 receptors, Suramin inhibits the acitivation of heterotrimeric G proteins in a variety of other GPCRs with varying potency. It prevents the association of heteromeric G proteins and therefore the receptors Guanine exchange functionality (GEF). With this blockade the GDP will not release from the Gα subunit so it can not be replaced by a GTP and become activated. This has the effect of blocking downstream G protein mediated signaling of various GPCR proteins including Rhodopsin, the A1 Adenosine receptor, and the D2 dopamine receptor.[11]
References
- ↑ The formula of suramin was kept secret by Bayer for commercial reasons. But it was elucidated and published in 1924 by Fourneau and his team of the Pasteur Institute, and it is only on this date that its exact chemical composition was known. (E. Fourneau, J. and Th. Tréfouël and J. Vallée (1924). "Sur une nouvelle série de médicaments trypanocides", C. R. Séances Acad. Sci. 178: 675.)
- ↑ Darsaud A, Chevrier C, Bourdon L, Dumas M, Buguet A, Bouteille B (January 2004). "Megazol combined with suramin improves a new diagnosis index of the early meningo-encephalitic phase of experimental African trypanosomiasis". Trop. Med. Int. Health 9 (1): 83–91. doi:10.1046/j.1365-3156.2003.01154.x. PMID 14728611.
- ↑ Anderson J, Fuglsang H (July 1978). "Further studies on the treatment of ocular onchocerciasis with diethylcarbamazine and suramin". Br J Ophthalmol 62 (7): 450–7. doi:10.1136/bjo.62.7.450. PMC 1043255. PMID 678497.
- ↑ Ahles TA, Herndon JE, Small EJ, et al. (November 2004). "Quality of life impact of three different doses of suramin in patients with metastatic hormone-refractory prostate carcinoma: results of Intergroup O159/Cancer and Leukemia Group B 9480". Cancer 101 (10): 2202–8. doi:10.1002/cncr.20655. PMID 15484217.
- ↑ http://medicalxpress.com/news/2013-03-drug-treatment-autism-symptoms-mouse.html
- ↑ Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. (september 2006). "International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy". Pharmacol Rev. 58 (3): 281–341. doi:10.1124/pr.58.3.3. PMID 16968944.
- ↑ Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Séguéla P, Voigt M, Humphrey PP. (march 2001). "International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits". Pharmacol Rev. 53 (1): 107–118. PMID 11171941.
- ↑ Wolner I, Kassack MU, Ullmann H, Karel A, Hohenegger M (October 2005). "Use-dependent inhibition of the skeletal muscle ryanodine receptor by the suramin analogue NF676". Br. J. Pharmacol. 146 (4): 525–33. doi:10.1038/sj.bjp.0706359. PMC 1751178. PMID 16056233.
- ↑ Erguven M, Akev N, Ozdemir A, Karabulut E, Bilir A (August 2008). "The inhibitory effect of suramin on telomerase activity and spheroid growth of C6 glioma cells". Med. Sci. Monit. 14 (8): BR165–73. PMID 18667993.
- ↑ Mastrangelo E, Pezzullo M, Tarantino D, Petazzi R, Germani F, Kramer D, Robel I, Rohayem J, Bolognesi M, Milani M (2012) Structure-based inhibition of norovirus RNA-dependent RNA-polymerases. J Mol Biol
- ↑ Beindl W, Mitterauer T, Hohenegger M, Ijzerman AP, Nanoff C, Freissmuth M. (August 1996). "Inhibition of receptor/G protein coupling by suramin analogues". ol. Pharmacology. 50 (2): 415–23. PMID 8700151.
External links
- Suramin bound to proteins in the PDB
- Drug information
- Suramin, drug information by JBC Online
- Suramin in treating patients with recurrent bladder cancer
- National Cancer Institute
| |||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||