Sulfur difluoride
From Wikipedia, the free encyclopedia
| Sulfur difluoride | |
|---|---|
 |
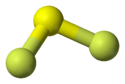 |
 | |
| IUPAC name sulfoxylic difluoride | |
| Identifiers | |
| CAS number | 13814-25-0 |
| PubChem | 139605 |
| ChemSpider | 123122 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | SF2 |
| Molar mass | 70.062 g/mol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Sulfur difluoride is an inorganic compound with the chemical formula SF2. It can be generated by the reaction of sulfur dichloride and potassium fluoride or mercury(II) fluoride at low pressures:
- SCl2 + 2 KF → SF2 + 2 KCl
- SCl2 + HgF2 → SF2 + HgCl2
The F-S-F bond angle is 98°, and the length of S-F bond is 159 pm.[1] The compound is highly unstable with respect to FSSF3. This unsymmetrical isomer of S2F4 is proposed to arise via insertion of SF2 into the S-F bond of a second molecule SF2:[2]
References
- ↑ Johnson, D. R.; Powell, F. X. (1969). "Microwave Spectrum and Structure of Sulfur Difluoride". Science 164 (3882): 950–1. doi:10.1126/science.164.3882.950. PMID 17775599.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0080379419.
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.