Sulfinamide
From Wikipedia, the free encyclopedia
Not to be confused with sulfonamide.
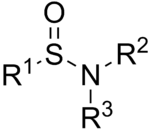
The general chemical structure of a sulfinamide
Sulfinamide is a functional group in organic chemistry containing a sulfur-oxygen double bond and a sulfur-nitrogen single bond. Sulfinamides are amides of sulfinic acid.
Chiral sulfinamides such as tert-butanesulfinamide, p-toluenesulfinamide[1][2] and 2,4,6-trimethylbenzenesulfinamide[3] are relevant to asymmetric synthesis.
References
- ↑ Fanelli, D. L.; Szewczyk, J. M.; Zhang, Y.; Reddy, G. V.; Burns, D. M.; Davis, F. A. (2000), "SULFINIMINES (THIOOXIMINE S-OXIDES): ASYMMETRIC SYNTHESIS OF METHYL (R)-(+)-β-PHENYLALANATE FROM (S)-(+)-N-(BENZYLIDENE)-p-TOLUENESULFINAMIDE", Org. Synth. 77: 50; Coll. Vol. 10: 47
- ↑ Ruano, J. L.; Alemán, J.; Parra, A.; Cid, M. B. (2007), "PREPARATION OF N-p-TOLYLSULFONYL-(E)-1-PHENYLETHYLIDENEIMINE", Org. Synth. 84: 129
- ↑ Ramachandar, T.; Wu, Y.; Zhang, J.; Davis, F. A. (2006), "(S)-(+)-2,4,6-TRIMETHYLBENZENESULFINAMIDE", Org. Synth. 83: 131
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.