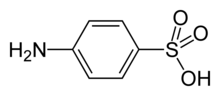
Sulfanilic acid
| Sulfanilic acid | ||
|---|---|---|
 | ||
 | ||
 | ||
| IUPAC name p-aminobenzenesulphonic acid | ||
| Other names Sulphanilic acid | ||
| Identifiers | ||
| CAS number | 121-57-3 | |
| PubChem | 8479 | |
| ChEBI | CHEBI:27500 | |
| Jmol-3D images | Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | C6H7NO3S | |
| Molar mass | 173.19 | |
| Density | 1.485 | |
| Melting point | 288 °C; 550 °F; 561 K | |
| Solubility in water | >20 g/l | |
| Acidity (pKa) | 3.01 | |
| Related compounds | ||
| Related sulfonic acids | Benzenesulfonic acid p-Toluenesulfonic_acid | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Sulfanilic acid (4-aminobenzenesulfonic acid ) is an off-white crystalline solid which finds application in quantitative analysis of nitrate and nitrite ions. The solid acid exists as a zwitterion, and has an unusually high melting point. [1]
Synthesis
Sulfanilic acid can be produced by sulfonation of aniline:[2]

Applications
As the compound readily form diazo compounds, it is used to make dyes and sulpha drugs. [1] This property is also used for the quantitative analysis of nitrate and nitrite ions by diazonium coupling reaction with N-(1-Naphthyl)ethylenediamine, resulting in an azo dye, and the concentration of nitrate or nitrite ions were deduced from the color intensity of the resulting red solution by colorimetry. [3]
ethylenediamine.png)
It is also used as a standard in combustion analysis.
References
- ↑ 1.0 1.1 "Sulphanilic acid". A Dictionary of Chemistry. Oxford University Press, 2000. Oxford Reference Online. Oxford University Press.
- ↑ Siegfried Hauptmann: Organische Chemie, 2nd Edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 511, ISBN 3-342-00280-8.
- ↑ G. H. Jerffery; J. Bassett; J. Mendham; R. C. Denney (1989). "Colorimetry and Spectrophotometry". Vogel's Textbook of Quantitative Chemical Analysis, 5th Edition. Longman. p. 702. ISBN 0-582-44693-7.