Stryker's reagent
| Stryker's reagent | ||
|---|---|---|
 | ||
| Identifiers | ||
| CAS number | 33636-93-0 | |
| PubChem | 12181933 | |
| Jmol-3D images | {{#if:C1=CC=C(C=C1)P(C2=CC=CC=C2)C3=CC=CC=C3.C1=CC=C(C=C1)P(C2=CC=CC=C2)C3=CC=CC=C3.C1=CC=C(C=C1)P(C2=CC=CC=C2)C3=CC=CC=C3.C1=CC=C(C=C1)P(C2=CC=CC=C2)C3=CC=CC=C3.C1=CC=C(C=C1)P(C2=CC=CC=C2)C3=CC=CC=C3.C1=CC=C(C=C1)P(C2=CC=CC=C2)C3=CC=CC=C3.[CuH].[CuH].[CuH].[CuH].[CuH].[CuH]|Image 1 | |
| ||
| Properties | ||
| Molecular formula | C108H96Cu6P6 | |
| Molar mass | 1961.036406 | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Stryker's reagent ([(PPh3)CuH]6),[1] also known as the Osborn complex, is a hexameric copper hydride ligated with triphenylphosphine. It is a brick red air-sensitive solid. Stryker's reagent is a mild source of hydride and is used in homogeneous catalysis of conjugate reduction reactions of enones, enoates, and related substrates.
Preparation and structure
The compound is prepared by hydrogenation of copper(I) tert-butoxide, generated in situ from copper(I) chloride and sodium tert-butoxide.[2] Other more convenient methods have been developed since its discovery.[3][4]
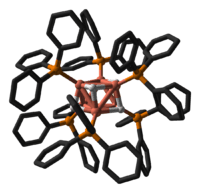
In terms of its structure, the compound is an octahedral cluster featuring Cu(PPh3) centres that are bonded by weak Cu---Cu interactions. Six of the eight faces are capped by hydride ligands.[5]
Applications in organic synthesis
The compound can effect highly regioselective conjugate reductions of various carbonyl derivatives including unsaturated aldehydes, ketones, and esters. This reagent was assigned as the "Reagent of the year" in 1991 for its functional group tolerance, high overall efficiency, and mild reaction conditions in the reduction reactions. Stryker's reagent is used in a catalytic amount where it is regenerated in the reaction in situ using a stoichiometric hydride source, often being molecular hydrogen or silanes. If stored under an inert atmosphere (e.g. argon, nitrogen) it has indefinite shelf life. Brief exposure to the oxygen does not destroy its activity significantly, although solvents used with Stryker's reagent should be rigorously degassed.[6]
Modifications to Stryker's reagent
Ligand-modified versions of Stryker's reagent have been reported. By changing the Ligand to e.g. P(O-iPr)3 the selectivity can be improved significantly.[7]
References
- ↑ Mahoney, W.S.; Brestensky, D.M.; Stryker, J.M. (1988). "Selective Hydride-mediated conjugate reduction of α,β-unsaturated Carbonyl Compounds Using [(Ph3P)CuH]6". J. Am. Chem. Soc. 110: 291–293. doi:10.1021/ja00209a048.
- ↑ Bezman, S. A.; Churchill, M. R.; Osborn, J. A.; Wormald, J. (1971). "Preparation and Crystallographic Characterization of a Hexameric Triphenylphosphinecopper Hydride Cluster". J. Am. Chem. Soc. 93 (8): 2063–2065. doi:10.1021/ja00737a045.
- ↑ O. Riant "Copper(I) hydride reagents and catalysts" Patai's Chemistry of Functional Groups, 2011, John Wiley & Sons. doi:10.1002/9780470682531.pat0448
- ↑ R. D. Stephens "Hydrido(Triphenylphosphine)Copper(I)" Inorganic Syntheses, 1979, vol. 19, pp. 87–89. doi:10.1002/9780470132500.ch17
- ↑ Raymond C. Stevens, Malcolm R. McLean, Robert Bau, Thomas F. Koetzle "Neutron diffraction structure analysis of a hexanuclear copper hydrido complex, H6Cu6[P(p-tolyl)3]6: an unexpected finding" J. Am. Chem. Soc. 1989, pp 3472–3473. doi:10.1021/ja00191a077
- ↑ John F. Daeuble and Jeffrey M. Stryker "Hexa-μ-hydrohexakis(triphenylphosphine)hexacopper" eEROS Encyclopedia of Reagents for Organic Synthesis, 2001. doi:10.1002/047084289X.rh011m
- ↑ Andrejs Pelss J. Org. Chem. 2009, 74, 7598-7601. doi:10.1021/jo9017588