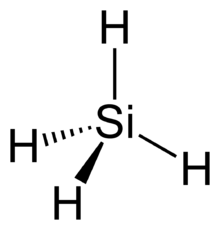
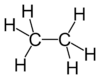
Structural analog
- For other uses of analog, see Analog (disambiguation).
In chemistry, a structural analog (structural analogue), also known as chemical analog or simply analog, is a compound having a structure similar to that of another one, but differing from it in respect of a certain component.[1][2][3]
It can differ in one or more atoms, functional groups, or substructures, which are replaced with other atoms, groups, or substructures. A structural analog can be imagined to be formed, at least theoretically, from the other compound.
Despite a high chemical similarity, structural analogs are not necessarily functional analogs and can have very different physical, chemical, biochemical, or pharmacological properties.[4]
In drug development either a large series of structural analogs of an initial lead compound are created and tested as part of a structure-activity relationship study[5] or a database is screened for structural analogs of a lead compound.[6]
Examples
| Carbon-Based | Silicon-Based |
|---|---|
See also
- Derivative (chemistry)
- Homolog, a compound of a series differing only by repeated units
- Functional analog, compounds with similar physical, chemical, biochemical, or pharmacological properties
- Transition state analog
References
- ↑ Willett, Peter, Barnard, John M. and Downs, Geoffry M. (1998). "Chemical Similarity Searching". Journal of Chemical Information and Computer Science 38: 983−996.
- ↑ A. M. Johnson, G. M. Maggiora (1990). Concepts and Applications of Molecular Similarity. New York: John Willey & Sons. ISBN 0-471-62175-7.
- ↑ N. Nikolova, J. Jaworska (2003). "Approaches to Measure Chemical Similarity - a Review". QSAR & Combinatorial Science 22 (9-10): 1006–1026. doi:10.1002/qsar.200330831.
- ↑ Martin, Yvonne C., Kofron, James L. and Traphagen, Linda M. (2002). "Do Structurally Similar Molecules Have Similar Biological Activity?". Journal of Medicinal Chemistry. 45(19): 4350–4358. doi:10.1021/jm020155c.
- ↑ Schnecke, Volker and Boström, Jonas (2006). "Computational chemistry-driven decision making in lead generation". Drug Discovery Today. 11(1-2): 43–50.
- ↑ Rester, Ulrich (2008). "From virtuality to reality - Virtual screening in lead discovery and lead optimization: A medicinal chemistry perspective". Current Opinion in Drug Discovery & Development 11 (4): 559–68. PMID 18600572.
External links
- Analoging in ChEMBL — a free web-service for finding structural analogs in ChEMBL.