Stille reaction
The Stille reaction, or the Migita-Kosugi-Stille coupling, is a chemical reaction widely used in organic synthesis which involves the coupling of an organotin compound (also known as organostannanes) with a variety of organic electrophiles via palladium-catalyzed coupling reaction.[1][2][3]

The R1 group attached to the trialkyltin is normally sp2-hybridized, including alkenes, and aryl groups; however, conditions have been devised to incorporate both sp3-hybridized groups, such as allylic and benzylic substituents, and sp-hybdrized alkynes. These organostannanes are also stable to both air and moisture, and many of these reagents are either commercially available or can be synthesized from literature precedent. However, these tin reagents tend to be highly toxic. X is typically a halide, such as Cl, Br, I, yet pseudohalides such as triflates and sulfonates and phopsphates can also be used.[4][5]
The groundwork for the Stille reaction was laid by Colin Eaborn, Toshihiko Migita, and Masanori Kosugi in 1976 and 1977, who explored numerous palladium catalyzed couplings involving organotin reagents. John Stille developed a much milder and more broadly applicable procedure in 1978.[6] Stille’s work on this area might have earned him a share of the 2010 Nobel Prize, which was awarded to Richard Heck, Ei-ichi Negishi, and Akira Suzuki for their work on the Heck, Negishi, and Suzuki coupling reactions. However, Stille died in the plane crash of United Airlines Flight 232 in 1989.[7]
Several reviews have been published on the Stille reaction.[2][8][9][10][11][12][13][14][15]
History
The first example of a palladium catalyzed coupling of aryl halides with organotin reagents was reported by Colin Eaborn in 1976 (A).[16] This reaction yielded from 7% to 53% of diaryl product. This process was expanded to the coupling of acyl chlorides with alkyl-tin reagents in 1977 by Toshihiko Migita, yielding 53% to 87% ketone product (B).[17]

Later in 1977, Migita published further work on the coupling of allyl-tin reagents with both aryl (C) and acyl (D) halides. The greater ability of allyl groups to migrate to the palladium catalyst allowed the reactions to be performed at lower temperatures. Yields for aryl halides ranged from 4% to 100%, and for acyl halides from 27% to 86%.[18][19]

After this groundwork was laid, Stille reported the coupling of a variety of alkyl tin reagents in 1978 with numerous aryl and acyl halides with much more mild reaction conditions, with much better yields (76%-99%).[20][20][21] Stille continued his work in the 1980’s on the synthesis of a multitude of ketones using this broad and mild process and elucidated a mechanism for this transformation.[17][22]

By the mid-1980s, over 65 papers on the topic of coupling reactions involving tin had been published, continuing to explore the substrate scope of this reaction. While initial research in the field focused on the coupling of alkyl groups, most future work involved the much more synthetically useful coupling of vinyl, alkenyl, aryl, and allyl organostannanes to halides. Due to these organotin reagent’s stability to air and their ease of synthesis, the Stille reaction became commonly used in organic synthesis.[9]
Mechanism
The mechanism of the Stille reaction is one of the most extensively studied pathways for coupling reactions.[12][23] The basic catalytic cycle, as seen below, involves an oxidative addition of a halide or pseudohalide (2) to a palladium catalyst (1), transmetalation of 3 with an organotin reagent (4), and reductive elimination of 5 to yield the coupled product (7) and the regenerated palladium catalyst (1).[24]

However, the detailed mechanism of the Stille coupling is extremely complex and can occur via numerous reaction pathways. Like other palladium-catalyzed coupling reactions, the active palladium catalyst is believed to be a 14-electron Pd(0) complex, which can be generated in a variety of ways. Use of an 18- or 16- electron Pd(0) source Pd(PPh3)4, Pd(dba)2 can undergo ligand dissociation to form the active species. Second, phosphines can be added to ligandless palladium(0). Finally, as pictured, reduction of a Pd(II) source (8) (Pd(OAc)2, PdCl2(MeCN)2, PdCl2(PPh3)2, BnPdCl(PPh3)2, etc.) by added phosphine ligands or organotin reagents is also common [6]
Oxidative Addition
For most sp2-hybridized organohalides, a concerted three-center oxidative addition to this 14-electron Pd(0) complex is proposed. This process gives the cis-tetravalent 16-electron Pd(II) species. It has been suggested the presence of anionic ligands, such as OAc, accelerate this step by the formation of [Pd(OAc)(PR3)n]-, making the palladium species more nucleophillic.[12][25]

In some cases, especially when an sp3-hybridized organohalide is used, an SN2 type mechanism tends to prevail, yet this is not as commonly seen in the literature.[12][25]

However, despite normally forming a cis-intermediate after a concerted oxidative addition, this product is in rapid equilibrium with its trans-isomer, which is thermodynamically more stable. This cis–trans isomerism is a complicated process which involves at least four concurrent mechanisms, two of which are autocatalzyed and two which are assisted by solvent association to the metal.[26][27]

There are multiple reasons why isomerization is favored here. First, a bulky ligand set is usually used in these processes, such as phosphines, and it is highly unfavorable for them to adopt a cis orientation relative to each other, resulting in isomerization to the more favorable trans product.[26][27] An alternative explanation for this phenomena, dubbed antisymbiosis or transphobia, is by invocation of the sdn model.[24][28] Under this theory, palladium is a hypervalent species. Hence R1 and the trans ligand, being trans to each other, will compete with one palladium orbital for bonding. This 4-electron 3-center bond is weakest when two strong donating groups are present, which heavily compete for the palladium orbital. Relative to any ligand normally used, the C-donor R1 ligand has a much higher trans effect. This trans influence is a measure of how competitive ligands trans to each other will compete for palladium’s orbital. The usual ligand set, phophines, and C-donors (R1) are both soft ligands, meaning that they will form strong bonds to palladium, and heavily compete with each other for bonding.[20][29] Since halides or pseudohalides are significantly more electronegative, their bonding with palladium will be highly polarized, with most of the electron density on the X group, making them low trans effect ligands. Hence, it will be highly favorable for R1 to be trans to X, since the R1 group will be able to form a stronger bond to the palladium.[24][28][29]

Transmetalation
The transmetalation of the trans intermediate from the oxidative addition step is believed to proceed via a variety of mechanisms depending on the substrates and conditions. The most common type of transmetalation for the Stille coupling involves an associative mechanism. This pathway implies that the organostannane, normally a tin atom bonded to an allyl, alkenyl, or aryl group, can coordinate to the palladium via one of these double bonds. This produces a fleeting pentavalent, 18-electron species, which can then undergo ligand detachment to form a square planar complex again. Despite the organostannane being coordinated to the palladium through the R2 group, R2 must be formally transferred to the palladium (the R2-Sn bond must be broken), and the X group must leave with the tin, completing the transmetalation. This is believed to occur through two mechanisms.[30]
First, when the organostannane initially adds to the trans metal complex, the X group can coordinate to the tin, in addition to the palladium, producing a cyclic transition state. Breakdown of this adduct results in the loss of R3Sn-X and a trivalent palladium complex with R1 and R2 present in a cis relationship. Another commonly seen mechanism involves the same initial addition of the organostannane to the trans palladium complex as seen above; however, in this case, the X group does not coordinate to the tin, producing an open transition state. After the α-carbon relative to tin attacks the palladium, the tin complex will leave with a net positive charge. In the scheme below, please note that the double bond coordinating to tin denotes R2, so any alkenyl, allyl, or aryl group. Furthermore, the X group can dissociate at any time during the mechanism and bind to the Sn+ complex at the end. Density functional theory calculations predict that an open mechanism will prevail if the 2 ligands remain attached to the palladium and the X group leaves, while the cyclic mechanism is more probable if a ligand dissociates prior to the transmetalation. Hence, good leaving groups such as triflates in polar solvents favor the former, while bulky phosphine ligands will favor the latter.[30]

A less common pathway for transmetalation is through a dissociative or solvent assisted mechanism. Here, a ligand from the tetravalent palladium species dissociates, and a coordinating solvent can add onto the palladium. When the solvent detaches, to form a 14-electron trivalent intermediate, the organostannane can add to the palladium, undergoing an open or cyclic type process as above.[30]
Reductive elimination step
In order for R1-R2 to reductively eliminate, these groups must occupy mutually cis coordination sites. Any trans-adducts must therefore isomerize to the cis intermediate or the coupling will be frustrated. A variety of mechanisms exist for reductive elimination and these are usually considered to be concerted.[12][31][32]
First, the 16-electron tetravalent intermediate from the transmetalation step can undergo unassisted reductive elimination from a square planar complex. This reaction occurs in two steps: first, the reductive elimination is followed by coordination of the newly formed sigma bond between R1 and R2 to the metal, with ultimate dissociation yielding the coupled product.[12][31][32]

The previous process, however, is sometimes slow and can be greatly accelerated by dissociation of a ligand to yield a 14-electron T shaped intermediate. This intermediate can then rearrange to form a Y-shaped adduct, which can undergo faster reductive elimination.[12][31][32]

Finally, an extra ligand can associate to the palladium to form an 18-electron trigonal bipyramidal structure, with R1 and R2 cis to each other in equatorial positions. The geometry of this intermediate makes it similar to the Y-shaped above.[12][31][32]

The presence of bulky ligands can also increase the rate of elimination. Ligands such as phophines with large bite angles cause steric repulsion between L and R1 and R2, resulting in the angle between L and the R groups to increase and the angle between R1 and R2 to hence decrease, allowing for quicker reductive elimination.[12][24]
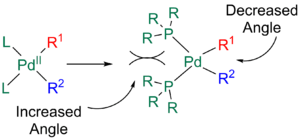
Kinetics
The rate at which organostannanes transmetalate with palladium catalysts is shown below. Sp2-hybridized carbon groups attached to tin are the most commonly used coupling partners, and sp3-hybridized carbons require harsher conditions and terminal alkynes may be coupled via a C-H bond through the Sonogashira reaction.
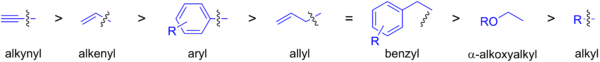
As the organic tin compound, a trimethylstannyl or tributylstannyl compound is normally used. Although trimethylstannyl compounds show higher reactivity compared with tributylstannyl compounds and have much similar 1H-NMR stretches, the toxicity of the former much larger.[33]
Optimizing which ligands are best at carrying out the reaction with high yield and turnover rate can be difficult. This is because the oxidative addition requires an electron rich metal, hence favoring electron donating ligands. However, an electron deficient metal is more favorable for the transmetalation and reductive elimination steps, making electron withdrawing ligands the best here. Therefore, the optimal ligand set heavily depends on the individual substrates and conditions used. These can change the rate determining step, as well as the mechanism for the transmetalation step.[34]
Normally, ligands of intermediate donicity, such as phosphines, are utilized. Rate enhancements can be seen when moderately electron-poor ligands, such as tri-2-furylphosphine or triphenylarsenine are used. Likewise, ligands of high donor number can slow down or inhibit coupling reactions.[34]<ref name=name=68>http://www.chem.harvard.edu/groups/myers/handouts/11_Stille.pdf</ref>
These observations imply that normally, the rate-determining step for the Stille reaction is transmetalation.<ref name=name=68>http://www.chem.harvard.edu/groups/myers/handouts/11_Stille.pdf</ref>
Additives
The most common additive to the Stille reaction is stoichiometric or co-catalytic copper(I), specifically copper iodide, which can greatly enhance rates up by >103 fold. While the exact mechanism of this rate enhancement is unknown, it has been theorized that in polar solvents copper transmetalate with the organostannane. The resulting organocuprate reagent could then transmetalate with the palladium catalyst. Furthermore, in ethereal solvents, the copper could also facilitate the removal of a phosphine ligand, activating the Pd center.[10][35][36][37][38]
Lithium chloride has been found to be a powerful rate accelerant in cases where the X group dissociates off of the palladium (i.e. the open mechanism). The chloride ion is believed to either displace the X group on the palladium making the catalyst more active for transmetalation or by coordination to the Pd(0) adduct to accelerate the oxidative addition. Also, LiCl salt enhances the polarity of the solvent, making it easier for this normally anionic ligand (–Cl, –Br, –OTf, etc) to leave. This additive is necessary when a solvent like THF is used; however, utilization of a more polar solvent, such as NMP, can replace the need for this salt additive. However, when the coupling’s transmetalation step proceeds via the cyclic mechanism, addition of lithium chloride can actually decrease the rate. As in the cyclic mechanism, a neutral ligand, such as phosphine, must dissociate instead of the anionic X group.[11][39]
Finally, fluoride ions, such as cesium fluoride, have also been found to have two useful effects on the catalytic cycle. First, fluouride can increase the rates of reactions of organotriflates, possibly by the same effect as lithium chloride. Furthermore, fluoride ions can act as scavengers for tin byproducts, making them easier to remove via filtration.[37]
Competing Side Reactons
The most common side reactivity associated with the Stille reaction is homocoupling of the stannane reagents to form an R2-R2 dimer. It is believed to proceed through two possible mechanism. First, reaction of two equivalents of organostannane with the Pd(II) precatalyst will yield the homocoupled product after reductive elimination. Second, the Pd(0) catalyst can undergo a radical process to yield the dimer. The organostannane reagent used is traditionally tetravalent at tin, normally consisting of the sp2-hybridized group to be transferred and three “non-transferable” alkyl groups. As seen above, alkyl groups are normally the slowest at migrating onto the palladium catalyst.[11]

It has also been found that at temperatures as low as 50 °C, aryl groups on both palladium and a coordinated phosphine can exchange. While normally not detected, they can be a potential minor product in many cases.[11]

Finally, a rather rare and exotic side reaction is known as cine substitution. Here, after initial oxidative addition of an aryl halide, this Pd-Ar species can insert across a vinyl tin double bond. After β-hydride elimination, migratory insertion, and protodestannyltion, a 1,2-disubstituted olefin can be synthesized.[11]

Numerous other side reactions can occur, and these include E/Z isomerization, which can potentially be a problem when an alkenylstannane is utilized. The mechanism of this transformation is currently unknown. Normally, organostannanes are quite stable to hydrolysis, yet when very electron-rich aryl stannanes are used, this can become a significant side reaction.[11]
Scope
Electrophile
Vinyl halides are common coupling partners in the Stille reaction, and reactions of this type are found in numerous natural product total syntheses. Normally, vinyl iodides and bromides are used. Vinyl chlorides are insufficiently reactive toward oxidative addition to Pd(0). Iodides are normally preferred: they will typically react faster and under milder conditions than will bromides. This difference is demonstrated below by the selective coupling of a vinyl iodide in the presence of a vinyl bromide.[11]
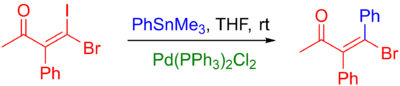
Normally, the stereochemistry of the alkene is retained throughout the reaction, except under harsh reaction conditions. A variety of alkenes may be used, and these include both α- and β-halo-α,β unsaturated ketones, esters, and sulfoxides (which normally need a copper (I) additive to proceed), and more (see example below).[40] Vinyl triflates are also sometimes used. Some reactions require the addition of LiCl and others are slowed down, implying that two mechanistic pathways are present.[11]

Another class of common electrophiles are aryl and heterocyclic halides. As for the vinyl substrates, bromides iodides are more common despite their greater expense. A multitude of aryl groups can be chosen, including rings substituted with electron donating substituents, biaryl rings, and more. Halogen-substituted heterocycles have also been used as coupling partners, including pyridines, furans, thiophenes, thiazoles, indoles, imidazoles, purines, uracil, cytosines, pyrimidines, and more (See below for table of heterocycles; halogens can be substituted at a variety of positions on each).[11]

Below is an example of the use of Stille coupling to build complexity on heterocycles of nucleosides, such as purines.[41]

Aryl triflates and sulfonates are also couple to a wide variety of organostannane reagents. Triflates tend to react comparably to bromides in the Stille reaction.[11]
Acyl chlorides are also used as coupling partners and can be used with a large range of organostannane, even alkyl-tin reagents, to produce ketones (see example below).[42] However, it is sometimes difficult to introduce acyl chloride functional groups into large molecules with sensitive functional groups. An alternative developed to this process is the Stille-carbonylative cross-coupling reaction, which introduces the carbonyl group via carbon monoxide insertion.[11]

Allylic, benzylic, and propargylic halides can also be coupled. While commonly employed, allylic halides proceed via an η3 transition state, allowing for coupling with the organostannane at either the α or γ position, occurring predominantly at the least substituted carbon (see example below).[43] Alkenyl epoxides (adjacent epoxides and alkenes) can also undergo this same coupling through an η3 transition state as, opening the epoxide to an alcohol. While allylic and benzylic acetates are commonly used, propargylic acetates are unreactive with organostannanes.[11]

Stannane
Organostannane reagents are extremely common, with a wide variety being commercially available.[44] Other stannanes may be synthesized via a variety of methods. First, addition of a Grignard or organolithium reagent to a trialkyltin halide will yield the organostannane. Palladium can also promote a radical addition of tin hydride to alkynes or alkenes. The benefits of using organotin reagents are that they are air and moisture stable (some reactions can even take place in water),[45] they can be synthesized, purified by chromatography, and store for long periods of time, and they are tolerant of most functional groups. However, they are heavily toxic, especially when using short chain alkyls.[11]
The use of vinylstannane, or alkenylstannane reagents is extremely common and widespread in use in the literature. In regards to limitations, both very bulky stannane reagents and stannanes with substitution on the α-carbon tend to react sluggishly or require optimization. For example, in the case below, the α-substituted vinylstannane only reacts with a terminal iodide due to steric hindrance.[46] However, despite these limitations, the number of couplings using alkenylstannane reagents is vast, and most examples on this page include reactions using them.[11]

Arylstannane reagents are also common and both electron donating and electron withdrawing groups actually increase the rate of the transmetalation. This again implies that two mechanisms of transmetalation can occur. The only limitation to these reagents are substituents at the ortho-position as small as methyl groups can decrease the rate of reaction. A wide variety of heterocycles (see Electrophile section) can also be used as coupling partners (see example with a thiazole ring below).[11][47]

While alkyl groups on the organostannane reagents are normally used as dummy, “non-transferable” ligands, there exist reported cases where these alkyl groups, especially benzyl groups, can be coupled at higher temperatures. Selectivity can be a problem if multiple types of alkyl groups are attached to the tin. The desired alkyl coupling partner must hence migrate to the palladium at a faster rate than the dummy ligands (see example below).[11][48]
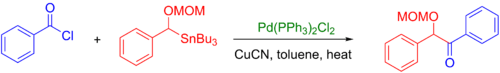
Other coupling partners include alkynylstannanes have also been used in Stille couplings and are the most reactive of all stannanes. However, they are not usually needed as terminal alkynes can couple directly to palladium catalysts through their C-H bond via Sonogashira coupling. Allylstannanes have been reported to have worked, yet difficulties arise, like with allylic halides, with the difficulty in control regioselectivity for α and γ addition. Distannane and acyl stannane reagents have also been used in Stille couplings.[11]
Applications
The Stille reaction has been used in the synthesis of a variety of polymers.[49][50]<ref name=name=57>Sun, S. S.; Lewis, J. E.; Zhang, J.; Jiang, X.; Zhang, C.; Matos, T.; Li, R.; Polym. Chem., 2010, 1, 663-669. (doi:10.1039/B9PY00324J)</ref> However, the most widespread use of the Stille reaction is its use in organic syntheses, and specifically, in the synthesis of natural products.
Natural Product Total Synthesis
Overman’s 19-step enantioselective total synthesis of quadrigemine C involves a double Stille cross metathesis reaction.[6][51] The complex organostannane is coupled onto two aryl iodide groups. After a double Heck cyclization, the product is achieved.

Panek’s 32 step enantioselective total synthesis of ansamycin antibiotic (+)-mycotrienol makes use of a late stage tandem Stille type macrocycle coupling. Here, the organostannane has two terminal tributyl tin groups attacked to an alkene. This organostannane “stiches” the two ends of the linear starting material into a macrocycle, adding the missing two methylene units in the process. After oxidation of the aromatic core with ceric ammonium nitrate (CAN) and deprotection with hydrofluoric acid yields the natural product in 54% yield for the 3 steps.[6][52]


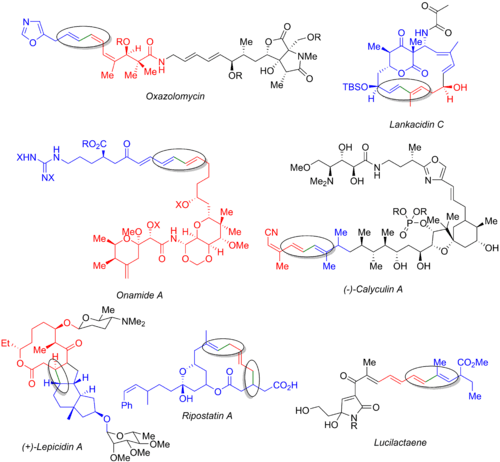
Variations
In addition to performing the reaction in a variety of organic solvents, conditions have been devised which allow for a broad range of Stille couplings in aqueous solvent.[15]
In the presence of Cu(I) salts, palladium-on-carbon has been shown to be an effective catalyst.[34][61]
In the realm of green chemistry a Stille reaction is reported taking place in a low melting and highly polar mixture of a sugar such as mannitol, a urea such as dimethylurea and a salt such as ammonium chloride[20] .[20] The catalyst system is tris(dibenzylideneacetone)dipalladium(0) with triphenylarsine:

Stille-Carbonylative Cross-Coupling
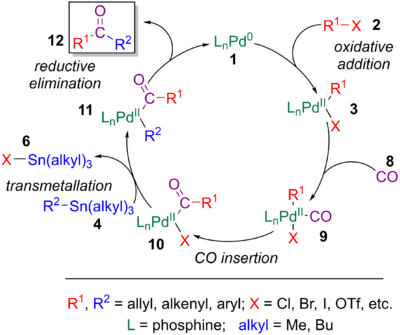
A common alteration to the Stille coupling is the encorporation of a carbonyl group between R1 and R2, serving as an efficient method to form ketones. This process is extremely similar to the initial exploration by Migita and Stille (see History) of coupling organostannane to acyl chlorides. However these moieties are not always readily available and can be difficult to form, especially in the presence of sensitive functional groups. Furthermore, controlling their high reactivity can be challenging. The Stille-carbonylative cross-coupling employs the same conditions as the Stille coupling, except with an atmosphere of carbon monoxide being used. The CO can coordinate to the palladium catalyst (9) after initial oxidative addition, followed by CO insertion into the Pd-R1 bond (10), resulting in subsequent reductive elimination to the ketone (12). The transmetalation step is normally the rate-determining step.[6][6]
Larry Overman and coworkers’ makes use of the Stille-carbonylative cross-coupling in their 20 step enantioselective total synthesis of Strychnine. The added carbonyl is later converted to a terminal alkene via a Wittig reaction allowing for the key tertiary nitrogen and the pentacyclic core to be formed via an aza-Cope-Mannich reaction.[6][62]
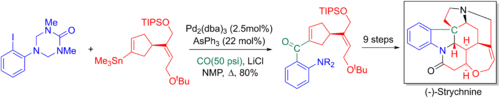
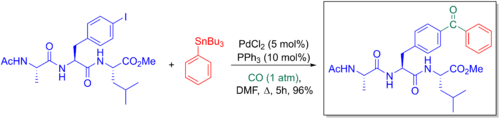
Giorgio Ortar et al. explored how the Stille-carbonylative cross-coupling could be used to synthesize benzophenone phosphores. These were embedded into 4-benzoyl-L-phenylalanine peptides and used for their photoaffinity labelling properties to explore various peptide-protein interactions.[6][63]
Louis Hegedus’ 16 step racemic total synthesis of Jatraphone involved a Stille-carbonylative cross-coupling as its final step to form the 11-membered macrocycle. In lieu of a hailde, a vinyl triflate is used here as the coupling partner.[6][64]

Stille-Kelly Coupling
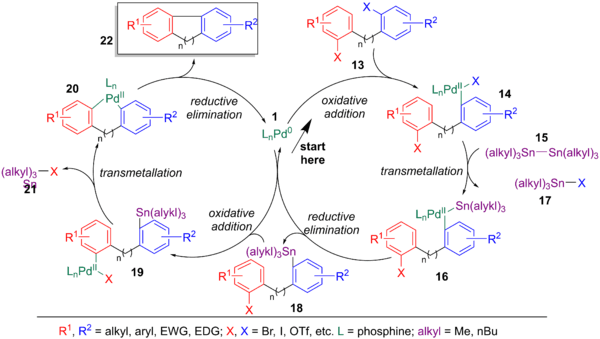
Using the seminal publication by Eaborn in 1976, which forms arylstannanes from arylhalides and distannanes, Kelly applied this process to the intramolecular coupling of arylhalides. This tandem stannylation/aryl halide coupling was used for the syntheses of a variety of dihydrophenanthrenes. Most of the internal rings formed are limited to 5 or 6 members, however some cases of macrocyclization have been reported. Unlike a normal Stille coupling, chlorine does not work as a halogen, possibly due to its lower reactivity in the halogen sequence (it’s shorter bond lend and stronger bond dissociation energy makes it more difficult to break via oxidative addition). Starting in the middle of the scheme below and going clockwise, the palladium catalyst (1) oxidatively adds to the most reactive C-X bond (13) to form 14, followed by transmetalation with distannane (15) to yield 16 and reductive elimination to yield an arylstannane (18). The regenerated palladium catalyst (1) can oxidative add to the second C-X bond of 18 to form 19, followed by intramolecular transmetalation to yield 20, followed by reductive elimination to yield the coupled product (22).[6]
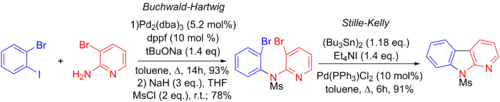
Jie Jack Lie et al. made use of the Stille-Kelly coupling in their synthesis of a variety of benzo[4,5]furopyridines ring systems. They invoke a three step process, involving a Buchwald-Hartwig amination, another palladium-catalyzed coupling reaction reaction, followed by an intramolecular Stille-Kelly coupling. Note that the aryl-iodide bond will oxidatively add to the palladium faster than either of the aryl-bromide bonds.[6][65]
See also
- Organotin chemistry
- Organostannane addition
- Palladium-catalyzed coupling reactions
- Suzuki reaction
- Negishi coupling
- Heck reaction
- Hiyama coupling
References
- ↑ Hartwig, J. F. Organotransition Metal Chemistry, from Bonding to Catalysis; University Science Books: New York, 2010. ISBN 189138953X
- ↑ 2.0 2.1 Stille, J. K. Angew. Chem. Int. Ed. Engl. 1986, 25, 508–524. (Review)
- ↑ Farina, V.; Krishnamurthy, V.; Scott, W. J. Org. React. 1998, 50, 1–652. (Review)
- ↑ Scott, W. J.; Crisp, G. T.; Stille, J. K. Organic Syntheses, Coll. Vol. 8, p.97 (1993); Vol. 68, p.116 (1990). (Article)
- ↑ Stille, J. K.; Echavarren, A. M.; Williams, R. M.; Hendrix, J. A. Organic Syntheses, Coll. Vol. 9, p.553 (1998); Vol. 71, p.97 (1993). (Article)
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 6.8 6.9 6.10 6.11 6.12 Kurti, L.; Czako, B. ‘‘Strategic Applications of Named Reactions in Organic Synthesis’’; Elsevier: Burlington, 2005.
- ↑ http://www.rsc.org/chemistryworld/Issues/2010/November/CarbonCouplersTakeThePrize.asp
- ↑ Mitchell, T. N. J. Organomet. Chem., 1986, 304, 1-16.
- ↑ 9.0 9.1 Mitchell, T. N. Synthesis, 1992, 803-815. (doi:10.1055/s-1992-26230)
- ↑ 10.0 10.1 Farina, V. Pure & Appl. Chem., 1996, 68, 73-78. (doi:10.1351/pac199668010073).
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 11.8 11.9 11.10 11.11 11.12 11.13 11.14 11.15 11.16 Farina, V.; Krishnamurthy, V.; Scott, W. J. ‘‘The Stille Reaction’’; Wiley: Online, 2004. (doi:10.1002/0471264180.or050.01).
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 Espinet, P.; Echavarren, A. M. Angew. Chem. Int. Ed., 2004, 43, 4704-4734.(doi:10.1002/anie.200300638)
- ↑ Pattenden, G.; Sinclair, D. J. J.Organomet. Chem., 2002, 653, 261-268.
- ↑ Kosugi, M.; Fugami, K. J. Organomet. Chem., 2002, 19, 10-16.
- ↑ 15.0 15.1 Pierre Genet, J.; Savignac, M. J. Organometa. Chem., 1999, 576, 305-317.
- ↑ Azarian, D.; Dua, S. S.; Eaborn, C.; Walton, D. R. M. J. Organomet. Chem., 1976, 117, C55-C57. (doi:10.1016/S0022-328X(00)91902-8)
- ↑ 17.0 17.1 Kosugi, M.; Shimizu, Y.; Migita, T. Chem. Lett., 1977, 6, 1423-1424. (doi:10.1246/cl.1977.1423)
- ↑ Kosugi, M.; Sasazawa, K.; Shikizu, Y.; Migita, T. Chem. Lett., 1977, 6, 301-302. (doi:10.1246/cl.1977.301)
- ↑ Kosugi, M.; Shimizu, Y.; Migita, T. J. Organomet. Chem., 1977, 129, C36-C38. (doi:10.1016/S0022-328X(00)92505-1)
- ↑ 20.0 20.1 20.2 20.3 20.4 Kosugi, M. et al. Chem. Letters 1977, 301.
- ↑ Milstein, D.; Stille, J. K. J. Am. Chem. Soc., 1978, 100, 3636-3638. (doi:10.1021/ja00479a077)
- ↑ Milstein, D.; Stille, J. K. J. Org. Chem., 1979, 44, 1613-1618. (doi:10.1021/jo01324a006)
- ↑ Casado, A. L.; Espinet, P.; Gallego, A. M. J. Am, Chem. Soc., 2000, 122, 11771-11782. (doi:10.1021/ja001511o)
- ↑ 24.0 24.1 24.2 24.3 Crabtree, R. H. The Organometallic Chemistry of the Transition Metals, 5th ed.; Wiley: New York, 2009.
- ↑ 25.0 25.1 Perez-Temprano, M. H.; Gallego, A. M.; Casares, J. A.; Espinet, P. Organometallics, 2011, 30, 611-617. (doi:10.1021/om100978w).
- ↑ 26.0 26.1 Minniti, D. Inorg. Chem, 1994, 33, 2631-2634.(doi:10.1021/ic00090a025).
- ↑ 27.0 27.1 Casado, A. L.; Espinet, P. Organometallics, 1998, 17, 954-959. (doi:10.1021/om9709502).
- ↑ 28.0 28.1 Landis, C. R.; Firman, T. K.' Root, D. M.; Cleveland, T. J. Am. Chem. Soc., 1998, 120, 1842-1854. (doi:10.1021/ja9710114).
- ↑ 29.0 29.1 Pearson, R. G. Inorg. Chem, 1973, 12, 712-713.(doi:10.1021/ic50121a052).
- ↑ 30.0 30.1 30.2 Garcia-Melchor, M.; Braga, A. A. C.; Lledos, A.; Ujaque, G.; Maseras, F. Acc. Chem. Res., 2013, 46, 2626-2634. (doi:10.1021/ar400080r)
- ↑ 31.0 31.1 31.2 31.3 Gillie, A.; Stille, J. K. J. Am. Chem. Soc., 1980, 102, 4933-4941. (doi:10.1021/ja00535a018).
- ↑ 32.0 32.1 32.2 32.3 Brown, J. M.; Cooley, N. A. Chem. Rev., 1988, 88, 1031-1046. (doi:10.1021/cr00089a003).
- ↑ McKillop, A.; Abel, E. W.; Stone, F. G. A.; Wilkinson, G. Comprehensive Organometallic Chemistry II, Elsevier Scientific: Oxford, 1995.
- ↑ 34.0 34.1 34.2 Farina, V.; J. Am. Chem. Soc., 1991, 113, 9585-9595. (doi:10.1021/ja00025a025).
- ↑ Liebeskind, L. S.; Fengl, R. W. J. Org. Chem., 1990, 55', 5359-5364. (doi:10.1021/jo00306a012).
- ↑ Farina, V.; Kapadia, S.; Brishnan, B.; Wang, C.; Liebeskind, L. S. J, Org. Chem, 1994, 59, 5905-5911. (doi:10.1021/jo00099a018).
- ↑ 37.0 37.1 Mee, S. P. H.; Lee, V.; Baldwin, J. E. Angew. Chem. Int. Ed., 2004, 43, 1132-1136.
- ↑ Liebeskind, L. S.; Peña-Cabrera, E. Organic Syntheses, Coll. Vol. 10, p.9 (2004); Vol. 77, p.135 (2000). (Article)
- ↑ Scott, W. J.; Stille, J. K. J. Am. Chem. Soc., 1986, 108, 3033-3040. (doi:10.1021/ja00271a037).
- ↑ Johnson, C. R.; Adams, J. P.; Braun, M.P.; Senanayake, C. B. W. Tet. Lett., 1992, 33, 919-922. (doi:10.1016/S0040-4039(00)91576-4)
- ↑ Nair, V.; Turner, G. A.; Chamberlain, S. D. J. Am. Chem. Soc., 1987, 109, 7223-7224. (doi:10.1021/ja00257a071).
- ↑ Jousseaume, B.; Kwon, W.; Verlhac, J. B.; Denat, F.; Dubac, J. Synlett, 1993, 117-118. (doi:10.1055/s-1993-22368)
- ↑ Sheffy, F. K.; Godschalx, J. P.; Stille, J. K. J. Am. Chem. Soc., 1984, 106, 4833-4840. (doi:10.1021/ja00329a032)
- ↑ http://www.sigmaaldrich.com/chemistry/chemistry-products.html?TablePage=16246425
- ↑ Wolf, C.; Lerebours, R. J. Org. Chem., 2003,68 7551-7554. (doi:10.1021/jo0347056).
- ↑ Crisp, G.T.; Glink, P. T. Tetrahedron, 1994, 50, 2623. (doi:10.1016/S0040-4020(01)86978-7)
- ↑ Bailey, T. R. Tet. Lett., 1986, 27, 4407. (doi:10.1016/S0040-4039(00)84964-3).
- ↑ Nativi, C.; Ricci, A.; Taddei, M. Tet. Lett., 1990, 31, 2637. (doi:10.1016/0040-4039(90)80147-E).
- ↑ Bao, Z.; Chan, W.; Yu, L. Chem. Mater., 1993, 5, 2-3. (doi:10.1021/cm00025a001).
- ↑ Bao, Z.; Chan, W. K.; Yu, L. J. Am. Chem. Soc., 1995, 117, 12426-12435. (doi:10.1021/ja00155a007).
- ↑ Lebsack, A. D.; Link, J. T.; Overman, L. E.; Stearns, B. A. J. Am. Chem. Soc., 2002, 124, 9008-9009. (doi:10.1021/ja9743194)
- ↑ Masse, C. E.; Yang, M.; Solomon, J.; Panek, J. S. J. Am. Chem. Soc., 1998, 120, 4123-4134. (doi:10.1021/ja0267425)
- ↑ Martin, S. F.; Humphrey, J. M.; Ali, A.; Hillier, M. C. J. Am. Chem. Soc., 1999, 121, 866-867. (doi:10.1021/ja9829259)
- ↑ Kende, A. S.; Kawamura, K.; DeVita, R. J. J. Am. Chem. Soc., 1990, 112 4070-4072. (doi:10.1021/ja00166a072).
- ↑ Kende, A. S., Koch, K.; Dorey, G.; Kaldor, I.; Liu, K. J. Amer. Chem. Soc., 1993, 115, 9842-9843. (doi:10.1021/ja00074a078).
- ↑ Hong, C. Y, Kishi, Y. J. Am. Chem. Soc., 1991, 113, 9693-9694. (doi:10.1021/ja00025a056).
- ↑ Tanimoto, N.; Gerritz, S. W.; Sawabe, A.; Noda, T.; Filla, S. A.; Masamune, S. Angew. Chem. Int. Ed., 2003, 33, 673-675. (doi:10.1002/anie.199406731).
- ↑ Evans, D. A.; Black, W. C. J. Am. Chem. Soc., 1993, 115, 4497-4513. (doi:10.1021/ja00064a011).
- ↑ Tang, W.; Prusov, E. V. Org. Lett., 2012, 14 4690-4693. (doi:10.1021/ol302219x).
- ↑ Coleman, R. S.; Walczak, M. C.; Campbell, E. L. J. Am. Chem. Soc., 2005, 127, 16036-16039. (doi:10.1021/ja056217g).
- ↑ Renaldo, A. F.; Labadie, J. W.; Stille, J. K. Organic Syntheses, Coll. Vol. 8, p. 268 (1993); Vol. 67, p.86 (1989). (Article)
- ↑ Knight, S. D.; Overman, L. E.; Pairaudeau, G. J. Am. Chem. Soc., 1993, 115, 9293-9294. (doi:10.1021/ja00073a057)
- ↑ Monera, E.; Ortar, G. Biorg. Med. Chem. Lett., 2000, 10, 1815-1818. (doi:10.1016/S0960-894X(00)00344-9).
- ↑ Gyorkos, A. C.; Stille, J. K.; Hegedus, L. S. J. Am. Chem. Soc., 1990, 112, 8465-8472. (doi:10.1021/ja00179a035).
- ↑ Yue, W. S.; Li, J. J. Org. Lett., 2002, 4, 2201-2203. (doi:10.1021/ol0260425)
External links
- Stille reaction handout from the Myers group.
- Stille reaction at organic-chemistry.org
- Stille reaction – Synthetic protocols from organic-reaction.com