Stercobilin
| Stercobilin | ||
|---|---|---|
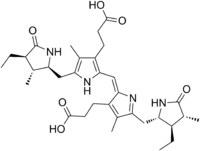 | ||
| IUPAC name 3-[(2E)-2-[ [3-(2-Carboxyethyl)-5- [(4-ethyl-3-methyl-5-oxo-pyrrolidin-2-yl) methyl]-4-methyl-1H-pyrrol-2-yl]methylidene]-5- [(3-ethyl-4-methyl-5-oxo-pyrrolidin-2-yl) methyl]-4-methyl-pyrrol-3-yl]propanoic acid | ||
| Identifiers | ||
| CAS number | 34217-90-8 | |
| PubChem | 5280818 | |
| MeSH | Stercobilin | |
| Properties | ||
| Molecular formula | C33H46N4O6 | |
| Molar mass | 594.742 | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Stercobilin is a tetrapyrrolic bile pigment and is one end-product of heme catabolism.[1][2] It is the chemical responsible for the brown color of human fecal material[1] and was originally isolated from feces in 1932.[2] Stercobilin (and related urobilin) can be used as a marker for biochemical identification of fecal pollution levels in rivers.[3]
Metabolism
Stercobilin results from breakdown of the heme moiety of hemoglobin found in erythrocytes.[2] Macrophages break down senescent erythrocytes and break the heme down into biliverdin, which rapidly reduces to free bilirubin.[1] Bilirubin binds tightly to plasma proteins (especially albumin) in the blood stream and is transported to the liver, where it is conjugated with one or two glucuronic acid residues into bilirubin diglucuronide, and secreted into the small intestine as bile.[4] In the small intestine, some bilirubin glucuronide is converted back to bilirubin via bacterial enzymes in the terminal ileum.[1] This bilirubin is further converted to colorless urobilinogen.[1] Any urobilinogen that remains in the colon is converted to stercobilinogen and finally oxidized to stercobilin, which is responsible for the brown color of human feces.[1] Stercobilin is then excreted in the feces.[4]
Role in disease
Obstructive jaundice
In obstructive jaundice, no bilirubin reaches the small intestine, meaning that there is no formation of stercobilinogen. The lack of stercobilin and other bile pigments causes feces to become clay-colored.[1]
Brown pigment gallstones
An analysis of two infants suffering from cholelithiasis observed that a substantial amount of stercobilin was present in brown pigment gallstones. This study suggested that brown pigment gallstones could form spontaneously in infants suffering from bacterial infections of the biliary tract.[5]
Role in treatment of disease
A 1996 study by McPhee et al. suggested that stercobilin and other related pyrrolic pigments — including urobilin, biliverdin, dimethyl ester, and xanthobilirubic acid — has potential to function as a new class of HIV-1 protease inhibitors when delivered at low micromolar concentrations. These pigments were selected due to a similar in shape to the successful HIV-1 protease inhibitor Merck L-700,417. Further research is suggested to study the pharmacological efficacy of these pigments.[6]
See also
- Bile pigment
- Bilirubin
- Biliverdin
- Heme
- Urobilin
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Boron W, Boulpaep E. Medical Physiology: a cellular and molecular approach, 2005. 984-986. Elsevier Saunders, United States. ISBN 1-4160-2328-3
- ↑ 2.0 2.1 2.2 Kay IT, Weimer M, Watson CJ (1963). “The formation in vitro of stercobilin from bilirubin.” J Biol Chem. 238:1122-3. PMID 14031566
- ↑ Lam CW, Lai CK, Chan YW (1998). "Simultaneous fluorescence detection of fecal urobilins and porphyrins by reversed-phase high-performance thin-layer chromatography". Clin Chem. 44(2):345-6. PMID 9474036
- ↑ 4.0 4.1 Seyfried H, Klicpera M, Leithner C, Penner E (1976). "Bilirubin metabolism". Wien Klin Wochenschr. 88:477-82. PMID 793184
- ↑ Treem WR, Malet PF, Gourley GR, Hyams JS (1989). “Bile and stone analysis in two infants with brown pigment gallstones and infected bile”. Gastroenterology 96(2 Pt 1):519-23. PMID 2642880
- ↑ McPhee F, Caldera P, Bemis G, McDonagh A, Kuntz I, and Craik C (1996). “Bile pigments as HIV-1 protease inhibitors and their effects on HIV-1 viral maturation and infectivity in vitro”. Biochem. J. 320: 681–686 PMID 8973584
External links
| |||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||