Stable nuclide
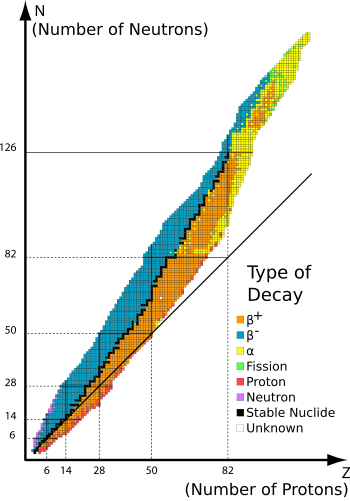
Stable nuclides are atomic species that are not radioactive - that is, they do not spontaneously undergo radioactive decay. When such nuclides are referred to in relation to specific elements, they are usually termed stable isotopes.
The 80 elements with one or more stable isotopes comprise a total of 254 nuclides that have not been known to decay using current equipment (see list at the end of this article). Only 90 nuclides (from the first 40 elements) are theoretically stable to any kind of decay save proton decay (see list of nuclides). An additional 164 are theoretically unstable to known types of decay, but no evidence of decay has ever been observed.
Of the 80 elements with one or more stable isotopes, twenty-six have only a single stable isotope, and are thus termed monoisotopic, and the rest have more than one stable isotope. One element (tin) has ten stable isotopes, the largest number known for an element.
Definition of stability, and naturally occurring nuclides
Most naturally occurring nuclides are stable (about 254; see list at the end of this article); and about 34 more (total of 288) are known radioactives with sufficiently long half-lives (also known) to occur "primordially." If the half-life of a nuclide is comparable to, or greater than, the Earth's age (4.5 billion years), a significant amount will have survived since the formation of the Solar System, and then is said to be primordial. It will then contribute in that way to the natural isotopic composition of a chemical element. Primordially present radioisotopes are easily detected with half-lives as short as 700 million years (e.g., 235U), although some primordial isotopes have been detected with half-lives as short as 80 million years (e.g., 244Pu). However, this is the present limit of detection, as the nuclide with the next-shortest half-life (niobium-92 with half-life 34.7 million years) has not yet been detected in nature.
Many naturally-occurring radioisotopes (another 51 or so, for a total of about 339) exhibit still shorter half-lives than 80 million years, but they are made freshly, as daughter products of decay processes of primordial nuclides (for example, radium from uranium) or from ongoing energetic reactions, such as cosmogenic nuclides produced by present bombardment of Earth by cosmic rays (for example, carbon-14 made from nitrogen).
Many isotopes that are classed as stable (i.e. no radioactivity has been observed for them) are predicted to have extremely long half-lives (sometimes as high as 1018 years or more). If the predicted half-life falls into an experimentally accessible range, such isotopes have a chance to move from the list of stable nuclides to the radioactive category, once their activity is observed. Good examples are bismuth-209 and tungsten-180 which were formerly classed as stable, but have been recently (2003) found to be alpha-active. However, such nuclides do not change their status as primordial when they are found to be radioactive.
Most stable isotopes in the earth are believed to have been formed in processes of nucleosynthesis, either in the 'Big Bang', or in generations of stars that preceded the formation of the solar system. However, some stable isotopes also show abundance variations in the earth as a result of decay from long-lived radioactive nuclides. These decay-products are termed radiogenic isotopes, in order to distinguish them from the much larger group of 'non-radiogenic' isotopes.
The so-called island of stability may reveal a number of long-lived or even stable atoms that are heavier (and with more protons) than lead.
Isotopes per element
Of the known chemical elements, 80 elements have at least one stable nuclide. These comprise the first 82 elements from hydrogen to lead, with the two exceptions, technetium (element 43) and promethium (element 61), that do not have any stable nuclides. As of December 2011, there were a total of 254 known "stable" nuclides. In this definition, "stable" means a nuclide that has never been observed to decay against the natural background. Thus, these elements have half lives too long to be measured by any means, direct or indirect.
Stable isotopes:
- 1 element (tin) has 10 stable isotopes
- 1 element (xenon) has 8 stable isotopes
- 4 elements have 7 stable isotopes apiece
- 8 elements have 6 stable isotopes apiece
- 10 elements have 5 stable isotopes apiece
- 9 elements have 4 stable isotopes apiece
- 5 elements have 3 stable isotopes apiece
- 16 elements have 2 stable isotopes apiece
- 26 elements have 1 single stable isotope.
These last 26 are thus called monoisotopic elements.[1] The mean number of stable isotopes for elements which have at least one stable isotope is 254/80 = 3.2.
"Magic numbers" and odd and even proton and neutron count
Stability of isotopes is affected by the ratio of protons to neutrons, and also by presence of certain "magic numbers" of neutrons or protons which represent closed and filled quantum shells. These quantum shells correspond to a set of energy levels within the shell model of the nucleus; filled shells, such as the filled shell of 50 protons for tin, confers unusual stability on the nuclide. As in the case of tin, a magic number for Z, the atomic number, tends to increase the number of stable isotopes for the element.
Just as in the case of electrons, which have the lowest energy state when they occur in pairs in a given orbital, nucleons (both protons and neutrons) exhibit a lower energy state when their number is even, rather than odd. This stability tends to prevent beta decay (in two steps) of many even-even nuclides into another even-even nuclide of the same mass number but lower energy (and of course with two more protons and two fewer neutrons), because decay proceeding one step at a time would have to pass through an odd-odd nuclide of higher energy. This makes for a larger number of stable even-even nuclides, up to three for some mass numbers, and up to seven for some atomic (proton) numbers. Conversely, of the 254 known stable nuclides, only five have both an odd number of protons and odd number of neutrons: hydrogen-2 (deuterium), lithium-6, boron-10, nitrogen-14, and tantalum-180m. Also, only four naturally occurring, radioactive odd-odd nuclides have a half-life over a billion years: potassium-40, vanadium-50, lanthanum-138, and lutetium-176. Odd-odd primordial nuclides are rare because most odd-odd nuclei are highly unstable with respect to beta decay, because the decay products are even-even, and are therefore more strongly bound, due to nuclear pairing effects.[2]
Yet another effect of the instability of an odd number of either type of nucleons, is that odd-numbered elements tend to have fewer stable isotopes. Of the 26 monoisotopic elements that have only a single stable isotope, all but one have an odd atomic number — the single exception to both rules being beryllium. All of these elements also have an even number of neutrons, with the single exception again being beryllium.
Nuclear isomers, including a "stable" one
The count of 254 known stable nuclides includes tantalum-180m, since even though its decay and instability is automatically implied by its notation of "metastable", still this has not yet been observed. All "stable" isotopes (stable by observation, not theory) are the ground states of nuclei, with the exception of tantalum-180m, which is a nuclear isomer or excited state (the ground state of this nucleus is radioactive with a very short half-life of 8 hours); but the decay of the excited nuclear isomer is extremely strongly forbidden by spin-parity selection rules. It has been reported experimentally by direct observation that the half-life of 180mTa to gamma decay must be more than 1015 years. Other possible modes of 180mTa decay (beta decay, electron capture and alpha decay) have also never been observed.
Still-unobserved decay

It is expected that some continual improvement of experimental sensitivity will allow discovery of very mild radioactivity (instability) of some isotopes that are considered to be stable today. For an example of a recent discovery, it was not until 2003 that bismuth-209 (the only naturally-occurring isotope of bismuth) was shown to be very mildly radioactive.[3] Prior to this discovery, there were theoretical predictions from nuclear physics that bismuth-209 would decay very slowly by alpha emission. These calculations were confirmed by the experimental observations in 2003.
Many "stable" nuclides are "metastable" inasmuch as they would release energy if a radioactive decay were to occur,[4] and are, in fact, expected to undergo very rare kinds of radioactive decay, including double-beta emission.
Ninety nuclides from the 40 elements with atomic numbers from one (hydrogen) through 40 are theoretically stable to any kind of nuclear decay—except for the theoretical possibility of proton decay - which has never been observed despite extensive searches for it.[citation needed]
The nuclides starting from with the isotope niobium-93 and extending to all higher atomic mass numbers, could theoretically experience spontaneous fission.[citation needed]
For processes other than spontaneous fission, other theoretical decay routes for heavier elements include:
- alpha decay - 70 heavy nuclides (the lightest two are cerium-142 and neodymium-143)
- double beta decay (including electron-positron conversion, and double positron decay) - 55 nuclides
- beta decay - tantalum-180m
- electron capture - tellurium-123, tantalum-180m
- double electron capture
- isomeric transition - tantalum-180m
- cluster decay and spontaneous fission - the 56 heavy nuclides (from niobium-93 to dysprosium-164)
These include all nuclides of mass 165 and greater. Argon-36 is presently the lightest known "stable" nuclide which is theoretically unstable.
The positivity of energy release in these processes means that they are allowed kinematically (they do not violate the conservation of energy) and, thus, in principle, can occur.[citation needed] They are not observed due to strong but not absolute suppression, by spin-parity selection rules (for beta decays and isomeric transitions) or by the thickness of the potential barrier (for alpha and cluster decays and spontaneous fission).
Summary table for numbers of each class of nuclides
This is a summary table from List of nuclides. Note that numbers are not exact, and may change slightly in the future, as nuclides are observed to be radioactive, or new half-lives are determined to some precision. Note that only the 254 have any claim to stability, but that only 90 nuclides from the first 40 elements are theoretically stable to any process but proton decay.
| Type of nuclide by stability class. | Number of nuclides in class (exact number may change). | Running total of nuclides in all classes to this point. | Notes on running total. |
|---|---|---|---|
| Theoretically stable to all but proton decay. | 90 | 90 | Includes first 40 elements. Proton decay yet to be observed. |
| Energetically unstable to one or more known decay modes, but no decay yet seen. Considered stable until radioactivity confirmed. | 164 | 254 | Spontaneous fission possible for "stable" nuclides > niobium-93. Other mechanisms possible for heavier nuclides. Total is the classically stable nuclides |
| Radioactive primordial nuclides. | 34 | 288 | Total primordials include Bi, U, Th, Pu, plus all stable nuclides. |
| Radioactive nonprimordial, but naturally occurring on Earth. | ~ 51 | ~ 339 | Cosmogenic nuclides from cosmic rays; daughters of radioactive primordials such as francium, etc. |
List of observationally-stable nuclides
In the list below, 90 nuclides have no predicted energetically-possible mode of decay, save proton decay. These are unmarked.
Other predicted (but not yet observed) modes of radioactive decay are noted as: A for alpha decay, B for beta decay, BB for double beta decay, E for electron capture, EE for double electron capture, and IT for isomeric transition. Because of the curve of binding energy, all nuclides from Z = 41 (niobium) and beyond, are theoretically unstable with regard to spontaneous fission SF (see list of nuclides for details), and many of the heavier nuclides are theoretically unstable to other processes as well.
- Hydrogen-1
- Hydrogen-2
- Helium-3
- Helium-4
- Lithium-6
- Lithium-7
- Beryllium-9
- Boron-10
- Boron-11
- Carbon-12
- Carbon-13
- Nitrogen-14
- Nitrogen-15
- Oxygen-16
- Oxygen-17
- Oxygen-18
- Fluorine-19
- Neon-20
- Neon-21
- Neon-22
- Sodium-23
- Magnesium-24
- Magnesium-25
- Magnesium-26
- Aluminium-27
- Silicon-28
- Silicon-29
- Silicon-30
- Phosphorus-31
- Sulfur-32
- Sulfur-33
- Sulfur-34
- Sulfur-36
- Chlorine-35
- Chlorine-37
- Argon-36 (EE)
- Argon-38
- Argon-40
- Potassium-39
- Potassium-41
- Calcium-40 (EE)
- Calcium-42
- Calcium-43
- Calcium-44
- Calcium-46 (BB)
- Scandium-45
- Titanium-46
- Titanium-47
- Titanium-48
- Titanium-49
- Titanium-50
- Vanadium-51
- Chromium-50 (EE)
- Chromium-52
- Chromium-53
- Chromium-54
- Manganese-55
- Iron-54 (EE)
- Iron-56
- Iron-57
- Iron-58
- Cobalt-59
- Nickel-58 (EE)
- Nickel-60
- Nickel-61
- Nickel-62
- Nickel-64
- Copper-63
- Copper-65
- Zinc-64 (EE)
- Zinc-66
- Zinc-67
- Zinc-68
- Zinc-70 (BB)
- Gallium-69
- Gallium-71
- Germanium-70
- Germanium-72
- Germanium-73
- Germanium-74
- Arsenic-75
- Selenium-74 (EE)
- Selenium-76
- Selenium-77
- Selenium-78
- Selenium-80 (BB)
- Bromine-79
- Bromine-81
- Krypton-78 (EE)
- Krypton-80
- Krypton-82
- Krypton-83
- Krypton-84
- Krypton-86 (BB)
- Rubidium-85
- Strontium-84 (EE)
- Strontium-86
- Strontium-87
- Strontium-88
- Yttrium-89
- Zirconium-90
- Zirconium-91
- Zirconium-92
- Zirconium-94 (BB)
- Niobium-93 (SF)
- Molybdenum-92 (EE)
- Molybdenum-94 (SF)
- Molybdenum-95 (SF)
- Molybdenum-96 (SF)
- Molybdenum-97 (SF)
- Molybdenum-98 (BB)
- Technetium - No stable isotopes
- Ruthenium-96 (EE)
- Ruthenium-98 (SF)
- Ruthenium-99 (SF)
- Ruthenium-100 (SF)
- Ruthenium-101 (SF)
- Ruthenium-102 (SF)
- Ruthenium-104 (BB)
- Rhodium-103 (SF)
- Palladium-102 (EE)
- Palladium-104 (SF)
- Palladium-105 (SF)
- Palladium-106 (SF)
- Palladium-108 (SF)
- Palladium-110 (BB)
- Silver-107 (SF)
- Silver-109 (SF)
- Cadmium-106 (EE)
- Cadmium-108 (EE)
- Cadmium-110 (SF)
- Cadmium-111 (SF)
- Cadmium-112 (SF)
- Cadmium-114 (BB)
- Indium-113 (SF)
- Tin-112 (EE)
- Tin-114 (SF)
- Tin-115 (SF)
- Tin-116 (SF)
- Tin-117 (SF)
- Tin-118 (SF)
- Tin-119 (SF)
- Tin-120 (SF)
- Tin-122 (BB)
- Tin-124 (BB)
- Antimony-121 (SF)
- Antimony-123 (SF)
- Tellurium-120 (EE)
- Tellurium-122 (SF)
- Tellurium-123 (E)
- Tellurium-124 (SF)
- Tellurium-125 (SF)
- Tellurium-126 (SF)
- Iodine-127 (SF)
- Xenon-124 (EE)
- Xenon-126 (EE)
- Xenon-128 (SF)
- Xenon-129 (SF)
- Xenon-130 (SF)
- Xenon-131 (SF)
- Xenon-132 (SF)
- Xenon-134 (BB)
- Caesium-133 (SF)
- Barium-132 (EE)
- Barium-134 (SF)
- Barium-135 (SF)
- Barium-136 (SF)
- Barium-137 (SF)
- Barium-138 (SF)
- Lanthanum-139 (SF)
- Cerium-136 (EE)
- Cerium-138 (EE)
- Cerium-140 (SF)
- Cerium-142 (A, BB)
- Praseodymium-141 (SF)
- Neodymium-142 (SF)
- Neodymium-143 (A)
- Neodymium-145 (A)
- Neodymium-146 (A, BB)
- Neodymium-148 (A, BB)
- Promethium - No stable isotopes
- Samarium-144 (EE)
- Samarium-149 (A)
- Samarium-150 (A)
- Samarium-152 (A)
- Samarium-154 (BB)
- Europium-153 (A)
- Gadolinium-154 (A)
- Gadolinium-155 (A)
- Gadolinium-156 (SF)
- Gadolinium-157 (SF)
- Gadolinium-158 (SF)
- Gadolinium-160 (BB)
- Terbium-159 (SF)
- Dysprosium-156 (A, EE)
- Dysprosium-158 (A, EE)
- Dysprosium-160 (A)
- Dysprosium-161 (A)
- Dysprosium-162 (A)
- Dysprosium-163 (SF)
- Dysprosium-164 (SF)
- Holmium-165 (A)
- Erbium-162 (A, EE)
- Erbium-164 (A, EE)
- Erbium-166 (A)
- Erbium-167 (A)
- Erbium-168 (A)
- Erbium-170 (A, BB)
- Thulium-169 (A)
- Ytterbium-168 (A, EE)
- Ytterbium-170 (A)
- Ytterbium-171 (A)
- Ytterbium-172 (A)
- Ytterbium-173 (A)
- Ytterbium-174 (A)
- Ytterbium-176 (A, BB)
- Lutetium-175 (A)
- Hafnium-176 (A)
- Hafnium-177 (A)
- Hafnium-178 (A)
- Hafnium-179 (A)
- Hafnium-180 (A)
- Tantalum-180m (A, B, E, IT)*
- Tantalum-181 (A)
- Tungsten-182 (A)
- Tungsten-183 (A)
- Tungsten-184 (A)
- Tungsten-186 (A, BB)
- Rhenium-185 (A)
- Osmium-184 (A, EE)
- Osmium-187 (A)
- Osmium-188 (A)
- Osmium-189 (A)
- Osmium-190 (A)
- Osmium-192 (A, BB)
- Iridium-191 (A)
- Iridium-193 (A)
- Platinum-192 (A)
- Platinum-194 (A)
- Platinum-195 (A)
- Platinum-196 (A)
- Platinum-198 (A, BB)
- Gold-197 (A)
- Mercury-196 (A, EE)
- Mercury-198 (A)
- Mercury-199 (A)
- Mercury-200 (A)
- Mercury-201 (A)
- Mercury-202 (A)
- Mercury-204 (A, BB)
- Thallium-203 (A)
- Thallium-205 (A)
- Lead-204 (A)
- Lead-206 (A)
- Lead-207 (A)
- Lead-208 (A)
Abbreviations:
A for alpha decay, B for beta decay, BB for double beta decay, E for electron capture, EE for double electron capture, IT for isomeric transition.
* Tantalum-180m is a "metastable isotope" meaning that it is an excited nuclear isomer of Ta-180. See isotopes of tantalum. However, the half life of this nuclear isomer is so long that it has never been observed to decay, and it thus occurs as an "observationally nonradioactive" primordial nuclide, as a minor isotope of tantalum. This is the only case of a nuclear isomer which has a half life so long that it has never been observed to decay. It is thus included in this list.
See also
- Table of nuclides
- List of nuclides (901 nuclides in order of stability, all with half-lives > one hour)
- Isotope geochemistry
- Radionuclide
- Mononuclidic element
- Primordial nuclide
- List of elements by stability of isotopes
- Periodic table
References
- ↑ Sonzogni, Alejandro. "Interactive Chart of Nuclides". National Nuclear Data Center: Brook haven National Laboratory. Retrieved 2008-06-06.
- ↑ Various (2002). Lide, David R., ed. Handbook of Chemistry & Physics (88th ed.). CRC. ISBN 0-8493-0486-5. OCLC 179976746. Retrieved 2008-05-23.
- ↑ "WWW Table of Radioactive Isotopes".
- ↑ AME2003 Atomic Mass Evaluation from the National Nuclear Data Center
Book references
- Various (2002). Lide, David R., ed. Handbook of Chemistry & Physics (88th ed.). CRC. ISBN 0-8493-0486-5. OCLC 179976746. Retrieved 2008-05-23.
External links
AlphaDelta: Stable Isotope fractionation calculator - http://www2.ggl.ulaval.ca/cgi-bin/isotope/generisotope.cgi
- National Isotope Development Center Reference information on isotopes, and coordination and management of isotope production, availability, and distribution
- Isotope Development & Production for Research and Applications (IDPRA) U.S. Department of Energy program for isotope production and production research and development
- Isosciences Use and development of stable isotope labels in synthetic and biological molecules