Specific activity
In nuclear sciences and technologies, "activity" is the SI quantity related to the phenomenon of natural and artificial radioactivity. The SI unit of "activity" is becquerel, Bq, while that of "specific activity" is Bq/kg. The old unit of "activity" was curie, Ci, while that of "specific activity" was Ci/g. For its use in radiochemistry, see radioactivity.
One can use the experimentally measured specific activity to calculate the half-life of an element.
Starting from the definition of the half-life (T1/2) - with, N0, atoms of an element, the number of atoms, N, remaining after time, t, is giving by:
Take the natural log of both sides:
Take the derivative with respect to time, t:
Multiply both sides by N:
Which is:
dN/dt represents the decay rate of atoms. The negative sign shows that the rate is negative, so the number of atoms is decreasing with time as you would expect. Rearranging terms:
Example: determine the half-life of Rb-87
Suppose you have a gram of rubidium-87 and with your Geiger counter, you get a count rate which, after taking solid angle effects into account, is consistent with a decay rate of 3200 decays per second; this would correspond to a specific activity of 3.2×106 Bq/kg. The atomic weight is of the rubidium is 87, so one gram is one 87th of a mole, or N=6.9×1021 atoms. Plugging in the numbers:
Formulation
First, radioactivity is expressed as the decay rate of a particular radionuclide with decay constant λ and the number of atoms N:
Next, mass of the radionuclide is given by
where m is mass number of the radionuclide and NA is Avogadro constant.
Specific radioactivity S is defined as radioactivity per unit mass of the radionuclide:
In addition, decay constant λ is related to the half-life T1/2 by the following equation:
Thus, specific radioactivity can also be described by
This equation is simplified by
When the unit of half-life converts a year
For example, specific radioactivity of radium 226 with a half-life of 1600 years is obtained by
This value derived from radium 226 was defined as unit of radioactivity known as Curie (Ci)
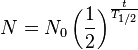
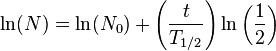
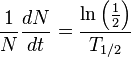
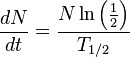
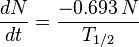
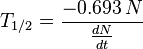
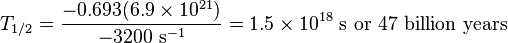
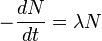
![{\frac {N}{N_{A}}}[{\text{mol}}]\times {m}[{\text{g }}{\text{mol}}^{{-1}}]](/2014-wikipedia_en_all_02_2014/I/media/2/e/9/0/2e90a2ae46f1aa08a5af26f1bb0d21c3.png)
![S[{\text{Bq/g}}]={\frac {\lambda N}{mN/N_{A}}}={\frac {\lambda N_{A}}{m}}](/2014-wikipedia_en_all_02_2014/I/media/f/5/0/e/f50ed54488bda90701e8a270a93eb318.png)
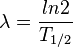
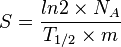
![S[{\text{Bq/g}}]\simeq {\frac {4.17\times 10^{{23}}}{T_{{1/2}}[s]\times m}}](/2014-wikipedia_en_all_02_2014/I/media/8/0/f/0/80f0cf96fff51cbee425d8e1cd6b1019.png)
![S[{\text{Bq/g}}]={\frac {ln2\times {N_{A}}}{T_{{1/2}}[s]\times {m}}}={\frac {ln2\times {N_{A}}}{T_{{1/2}}[year]\times 365\times 24\times 60\times 60\times m}}\simeq {\frac {1.32\times 10^{{16}}}{T_{{1/2}}[year]\times m}}](/2014-wikipedia_en_all_02_2014/I/media/b/5/e/a/b5eae8f24d86830045e458f3b98eb90d.png)
![{\frac {1.32\times 10^{{16}}}{1600[year]\times 226}}\simeq {3.7}\times 10^{{10}}[{\text{Bq/g}}]](/2014-wikipedia_en_all_02_2014/I/media/c/c/3/7/cc376442a03078d8b61bf6373dd9910c.png)