Somatic-cell nuclear transfer
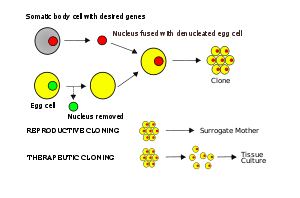
The process
The nucleus of a somatic cell is removed and kept, and the host's egg cell nucleus is removed and discarded. Now we have a lone nucleus and an empty (or deprogrammed) egg cell. The lone nucleus is then fused with the 'deprogrammed' egg cell. After being inserted into the egg, the lone (somatic-cell) nucleus is reprogrammed by the host egg cell. The egg, now containing the somatic cell's nucleus, is stimulated with a shock and will begin to divide. After many mitotic divisions, this single cell forms a blastocyst (an early stage embryo with about 100 cells) with almost identical DNA to the original organism. The technique of transferring a nucleus from a somatic cell into an egg that produced Dolly was an extension of experiments that had been ongoing for over 40 years. In the simplest terms, the technique used to produce Dolly the sheep – somatic-cell nuclear transplantation cloning – involves removing the nucleus of an egg and replacing it with the diploid nucleus of a somatic cell.
SCNT in stem cell research
Some researchers use SCNT in stem cell research. The aim of carrying out this procedure is to obtain stem cells that are genetically matched to the donor organism.
Embryonic stem cells are new, unspecialized cells that are able to be produced into a specialized cell that can replace another cell that has been lost in the body.

A potential use of genetically-customized stem cells would be to create cell lines that have genes linked to the particular disease. For example, if a person with Parkinson's disease donated his or her somatic cells, then the stem cells resulting from SCNT would have genes that contribute to Parkinson's disease. In this scenario, the disease specific stem cell lines would be studied in order to better understand the disease.[1]
In another scenario, genetically-customized stem cell lines would be generated for cell-based therapies to transplant to the patient. The resulting cells would be genetically identical to the somatic-cell donor, thus avoiding any complications from immune system rejection.[1][2]
Only a handful of the labs in the world are currently using SCNT techniques in human stem cell research. In the United States, scientists at the Harvard Stem Cell Institute, the University of California San Francisco, the Oregon Health & Science University,[3] Stemagen (La Jolla, CA) and possibly Advanced Cell Technology are currently researching a technique to use somatic-cell nuclear transfer to produce embryonic stem cells.[4] In the United Kingdom, the Human Fertilisation and Embryology Authority has granted permission to research groups at the Roslin Institute and the Newcastle Centre for Life.[5] SCNT may also be occurring in China.[6]
In 2005, a South Korean research team led by Professor Hwang Woo-suk, published claims to have derived stem cell lines via SCNT,[7] but supported those claims with fabricated data.[8] Recent evidence has proved that he in fact created a stem cell line from a parthenote.[9][10]
In May 2013, a group from the Oregon Health & Science University reported on the creation of human embryonic stem cell lines derived through SCNT.[3]
The impetus for SCNT-based stem cell research has been decreased by the development and improvement of alternative methods of generating stem cells. Methods to reprogram normal body cells into pluripotent stem cells were developed in humans in 2007. The following year, this method achieved a key goal of SCNT-based stem cell research: the derivation of pluripotent stem cell lines that have all genes linked to various diseases.[11] Some scientists working on SCNT-based stem cell research have recently moved to the new methods of induced pluripotent stem cells.
SCNT in reproductive cloning

This technique is currently the basis for cloning animals (such as the famous Dolly the sheep),[12] and in theory could be used to clone humans. However, most researchers believe that in the foreseeable future it will not be possible to use this technique to produce a human clone that will develop to term. It remains a possibility, though critical adjustments will be required to overcome current limitations during early embryonic development in human SCNT.[13][14]
Interspecies Nuclear Transfer
Interspecies nuclear transfer is a means of somatic cell nuclear transfer used to facilitate the rescue of endangered, or even to restore species after their extinction. The technique is similar to SCNT cloning which typically is between domestic animals and rodents, or where there is a ready supply of oocytes and surrogate animals. However, the cloning of highly endangered or extinct species requires the use of an alternative method of cloning. Interspecies nuclear transfer utilizes a host and donor of two different organisms that are closely related species and within the same genus. In 2000, Robert Lanza was able to produce a cloned fetus of a Gaur, Bos taurus combining it successfully with a domestic cow, Bos Taurus. [15]
Limitations
Stresses placed on both the egg cell and the introduced nucleus are enormous, leading to a high loss in resulting cells. For example, Dolly the sheep was born after 277 eggs were used for SCNT, which created 29 viable embryos. Only three of these embryos survived until birth, and only one survived to adulthood.[12] As the procedure currently cannot be automated, but has to be performed manually under a microscope, SCNT is very resource intensive. The biochemistry involved in reprogramming the differentiated somatic cell nucleus and activating the recipient egg is also far from understood.
In SCNT, not all of the donor cell's genetic information is transferred, as the donor cell's mitochondria that contain their own mitochondrial DNA are left behind. The resulting hybrid cells retain those mitochondrial structures which originally belonged to the egg. As a consequence, clones such as Dolly that are born from SCNT are not perfect copies of the donor of the nucleus.
Controversy
Proposals to use nucleus transfer techniques in human stem cell research raise a set of concerns beyond the moral status of any created embryo. These have led to some individuals and organizations who are not opposed to human embryonic stem cell research to be concerned about, or opposed to, SCNT research.[16][17][18]
One concern is that blastula creation in SCNT-based human stem cell research will lead to the reproductive cloning of humans. Both processes use the same first step: the creation of a nuclear transferred embryo, most likely via SCNT. Those who hold this concern often advocate for strong regulation of SCNT to preclude implantation of any derived products for the intention of human reproduction,[19] or its prohibition.[16]
A second important concern is the appropriate source of the eggs that are needed. SCNT requires human eggs, which can only be obtained from women. The most common source of these eggs today are eggs that are produced and in excess of the clinical need during IVF treatment. This is a minimally invasive procedure, but it does carry some health risks, such as ovarian hyperstimulation syndrome.
One vision for successful stem cell therapies is to create custom stem cell lines for patients. Each custom stem cell line would consist of a collection of identical stem cells each carrying the patient's own DNA, thus reducing or eliminating any problems with rejection when the stem cells were transplanted for treatment. For example, to treat a man with Parkinson's disease, a cell nucleus from one of his cells would be transplanted by SCNT into an egg cell from an egg donor, creating a unique lineage of stem cells almost identical to the patient's own cells. (There would be differences. For example, the mitochondrial DNA would be the same as that of the egg donor. In comparison, his own cells would carry the mitochondrial DNA of his mother.)
Potentially millions of patients could benefit from stem cell therapy, and each patient would require a large number of donated eggs in order to successfully create a single custom therapeutic stem cell line. Such large numbers of donated eggs would exceed the number of eggs currently left over and available from couples trying to have children through assisted reproductive technology. Therefore, healthy young women would need to be induced to sell eggs to be used in the creation of custom stem cell lines that could then be purchased by the medical industry and sold to patients. It is so far unclear where all these eggs would come from.
Stem cell experts consider it unlikely that such large numbers of human egg donations would occur in a developed country because of the unknown long-term public health effects of treating large numbers of healthy young women with heavy doses of hormones in order to induce hyperovulation (ovulating several eggs at once). Although such treatments have been performed for several decades now, the long-term effects have not been studied or declared safe to use on a large scale on otherwise healthy women. Longer-term treatments with much lower doses of hormones are known to increase the rate of cancer decades later. Whether hormone treatments to induce hyperovulation could have similar effects is unknown. There are also ethical questions surrounding paying for eggs. In general, marketing body parts is considered unethical and is banned in most countries. Human eggs have been a notable exception to this rule for some time.
To address the problem of creating a human egg market, some stem cell researchers are investigating the possibility of creating artificial eggs. If successful, human egg donations would not be needed to create custom stem cell lines. However, this technology may be a long way off.
Policies regarding human SCNT
SCNT involving human cells is currently legal for research purposes in the United Kingdom, having been incorporated into the Human Fertilisation and Embryology Act 1990 in 2001.[20] Permission must be obtained from the Human Fertilisation and Embryology Authority in order to perform or attempt SCNT.
In the United States, the practice remains legal, as it has not been addressed by federal law.[21] However, in 2002, a moratorium on United States federal funding for SCNT prohibits funding the practice for the purposes of research. Thus, though legal, SCNT cannot be federally funded.[22] American scholars have recently argued that because the product of SCNT is a clone embryo, rather than a human embryo, these policies are morally wrong and should be revised.[23]
In 2003, the United Nations adopted a proposal submitted by Costa Rica, calling on member states to "prohibit all forms of human cloning in as much as they are incompatible with human dignity and the protection of human life."[24] This phrase may include SCNT, depending on interpretation.
The Council of Europe's Convention on Human Rights and Biomedicine and its Additional Protocol to the Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine, on the Prohibition of Cloning Human Being appear to ban SCNT of human beings. Of the Council's 45 member states, the Convention has been signed by 31 and ratified by 18. The Additional Protocol has been signed by 29 member nations and ratified by 14.[25]
The UN is currently against all forms of human cloning.[citation needed]
See also
- Induced stem cells
- Stem cell research
- Stem cell controversy
- Embryogenesis
- In vitro fertilisation
- Cloning
- New Jersey legislation S1909/A2840
- Rejuvenation
References
- ↑ 1.0 1.1 Semb H (2005). "Human embryonic stem cells: origin, properties and applications". APMIS 113 (11–12): 743–50. doi:10.1111/j.1600-0463.2005.apm_312.x. PMID 16480446.
- ↑ Hadjantonakis AK, Papaioannou VE (July 2002). "Can mammalian cloning combined with embryonic stem cell technologies be used to treat human diseases?". Genome Biol. 3 (8): REVIEWS1023. doi:10.1186/gb-2002-3-8-reviews1023. PMC 139399. PMID 12186652.
- ↑ 3.0 3.1 Tachibana M (2013). "Human Embryonic Stem Cells Derived by Somatic Cell Nuclear Transfer". Cell. In Press. doi:10.1016/j.cell.2013.05.006.
- ↑ Elizabeth Weise, "Cloning race is on again", USA Today (January 17, 2006, retrieved October 6, 2006)
- ↑ "Dolly scientists' human clone bid", BBC News (September 28, 2004, retrieved October 6, 2006)
- ↑ Charles C. Mann, "The First Cloning Superpower", Wired (January 2003, retrieved October 6, 2006)
- ↑ Hwang WS, Roh SI, Lee BC, et al. (June 2005). "Patient-specific embryonic stem cells derived from human SCNT blastocysts". Science 308 (5729): 1777–83. doi:10.1126/science.1112286. PMID 15905366. (Retracted)
- ↑ Kennedy D (January 2006). "Editorial retraction". Science 311 (5759): 335. doi:10.1126/science.1124926. PMID 16410485.
- ↑ , Nature Stem Cell Blog.
- ↑ , The Scientist 19 June 2007
- ↑ Gretchen Vogel (December 2008). "Breakthrough of the year: Reprogramming Cells". Science 322 (5909): 1766–1767. doi:10.1126/science.322.5909.1766. PMID 19095902.
- ↑ 12.0 12.1 Campbell KH, McWhir J, Ritchie WA, Wilmut I (March 1996). "Sheep cloned by nuclear transfer from a cultured cell line". Nature 380 (6569): 64–6. doi:10.1038/380064a0. PMID 8598906.
- ↑ Revel M (2000). "Research on animal cloning technologies and their implications in medical ethics: an update". Med Law 19 (3): 527–43. PMID 11143888.
- ↑ Rhind SM, Taylor JE, De Sousa PA, King TJ, McGarry M, Wilmut I (November 2003). "Human cloning: can it be made safe?". Nat. Rev. Genet. 4 (11): 855–64. doi:10.1038/nrg1205. PMID 14634633.
- ↑ Lanza, Robert P.; Jose B. Cibelli, Francisca A. Diaz, Carlos T. Moraes,Peter W. Farin, Charlotte E. Farin, Carolyn J. Hammer, Michael D. West,and Philip Damiani (2000). "Cloning of an endangered species (Bos gaurus) using interspecies nuclear transfer". Cloning 2 (2): 79–90. Retrieved 10 December 2013.
- ↑ 16.0 16.1 Jeremy Rifkin. (February 18, 2002). "Fusion Biopolitics". The Nation. Retrieved on August 7, 2006.
- ↑ Sheryl Gay Stolberg, "Some for Abortion Rights Lean Right in Cloning Fight", New York Times (January 24, 2002)
- ↑ Lori B. Andrews, et al., Open Letter to US Senate on Human Cloning, (March 19, 2002)
- ↑ Lori B. Andrews et al. (March 19, 2002)."Open Letter to US Senators on Human Cloning and Eugenic Engineering". Retrieved on August 7, 2006
- ↑ Andy Coghlan, "Cloning opponents fear loopholes in new UK law", New Scientist (November 23, 2001, retrieved October 6, 2006)
- ↑ "Chapter 5: Legal and Policy Considerations. Cloning Human Beings" Report and Recommendations of the National Bioethics Advisory Commission, June 1997. Accessed 21 Oct 06
- ↑ Robertson, John A. (2010). "Embryo Stem Cell Research: Ten Years of Controversy". The Journal of Law, Medicine, & Ethics, vol. 38. n. 2 pp. 191–203.
- ↑ Cunningham, Thomas V. (2013). "What justifies the United States ban on federal funding for nonreproductive cloning?" Medicine, Health Care and Philosophy, vol. 16, n. 4, pp. 825-841.
- ↑ United Nations, "General Assembly Adopts United Nations Declaration on Human Cloning By Vote of 84-34-37", press release (August 3, 2005, retrieved October 6, 2006)
- ↑ Council of Europe, Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine (April 4, 1997, retrieved October 6, 2006); Council of Europe, Additional Protocol to the Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine, on the Prohibition of Cloning Human Being (January 12, 1998, retrieved October 6, 2006)
Further reading
- Wilmut I, Beaujean N, de Sousa PA, et al. (October 2002). "Somatic cell nuclear transfer". Nature 419 (6907): 583–6. doi:10.1038/nature01079. PMID 12374931.
- Kikyo N, Wolffe AP (January 2000). "Reprogramming nuclei: insights from cloning, nuclear transfer and heterokaryons". J. Cell. Sci. 113. ( Pt 1): 11–20. PMID 10591621.
- Tian XC, Kubota C, Enright B, Yang X (November 2003). "Cloning animals by somatic cell nuclear transfer—biological factors". Reprod. Biol. Endocrinol. 1 (1): 98. doi:10.1186/1477-7827-1-98. PMC 521203. PMID 14614770.
- Gurdon JB, Byrne JA, Simonsson S (September 2003). "Nuclear reprogramming and stem cell creation". Proc. Natl. Acad. Sci. U.S.A. 100. Suppl 1 (90001): 11819–22. doi:10.1073/pnas.1834207100. PMC 304092. PMID 12920185.
External links
- Research Cloning: Medical and scientific, legal and ethical aspects
- The Basics: Stem Cells and Public Policy The Century Foundation, June 2005
- "Research Cloning Basic Science", Center for Genetics and Society, (Last modified October 4, 2004, retrieved October 6, 2006)
- Cloning: present uses and promises National Institutes of Health, Paper giving background information on cloning in general and SCNT from The Office of Science Policy Analysis.
- Nuclear Transfer – Stem Cells or Somatic Cell Nuclear Transfer (SCNT) The International Society for Stem Cell Research
- The Hinxton Group: An International Consortium on Stem Cells, Ethics & Law