Sodium lauroyl sarcosinate
| Sodium lauroyl sarcosinate | ||
|---|---|---|
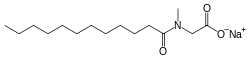 | ||
| IUPAC name sodium [dodecanoyl(methyl)amino]acetate | ||
| Identifiers | ||
| CAS number | 137-16-6 | |
| PubChem | 23668817 | |
| Jmol-3D images | {{#if:CCCCCCCCCCCC(=O)N(C)CC(=O)[O-].[Na+]|Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | C15H28NNaO3 | |
| Molar mass | 293.38 g mol−1 | |
| Melting point | 140 °C; 284 °F; 413 K | |
| Boiling point | 420 °C; 788 °F; 693 K | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Sodium lauroyl sarcosinate (INCI), also known as sarkosyl, is an ionic surfactant derived from sarcosine, used as a foaming and cleansing agent in shampoo, shaving foam and foam wash products.[1] In molecular biology experiments, sarkosyl is used to inhibit the initiation of DNA transcription.
This surfactant is amphiphilic due to the hydrophobic 14-carbon chain (lauroyl) and the hydrophilic carboxylate. Since the nitrogen atom is in an amide linkage, the nitrogen is not pH active and is neutral at all solutions regardless of pH. The carboxylate has a pKa of about 3.6 and is therefore negatively charged in solutions of pH greater than about 5.5.
pH-sensitive vesicles can be prepared using this surfactant with another cationic or water-insoluble amphiphiles such as 1-decanol.[2]
Addition of an mixture of equal parts of sodium lauroyl sarcosinate and the non-ionic surfactant sorbitan monolaurate (S20) to water led to the formation of micelle-like aggregates, even though neither surfactant formed micelles when present alone. Such aggregates can help carry other small molecules, such as drugs, through the skin.[3]
References
- ↑ Wallach, D.F.H; R. Mathur, G.J.M. Redziniak and J.F. Tranchant (1992). J. Soc.Cosmetic Chemists 43: 113–118.
- ↑ Akter, N; S. Radiman, F. Mohamed, I.A. Rahman, M.I.H. Reza (2011). Scientific Reports 1. doi:10.1038/srep00071.
- ↑ Karande, P; A.Jain, A. Arora, M.J.Ho, S. Mitragotri (2007). Eur. J. Pharm Sci. 31: 1–7.
- Ambühl, F; Bangerter, P.L. Luisi, P. Skrobal and H.J.Watzke (1993). Langmuir 9: 36–38.
- Ghosh, S; J.Dey (2011). J. Colloid and Interface Sci. 358: 208–216.
- Karande, P; A.Jain, A. Arora, M.J.Ho, S. Mitragotri (2007). Eur. J. Pharm Sci. 31: 1–7.