Sodium cyanide
| Sodium cyanide | |
|---|---|
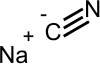 |
 |
| Identifiers | |
| CAS number | 143-33-9 |
| PubChem | 8929 |
| ChemSpider | 8587 |
| UN number | 1689 |
| ChEMBL | CHEMBL1644697 |
| RTECS number | VZ7525000 |
| Jmol-3D images | {{#if:[C-]#N.[Na+]|Image 1 |
| |
| |
| Properties | |
| Molecular formula | NaCN |
| Molar mass | 49.0072 g/mol |
| Appearance | white solid |
| Odor | faint almond-like |
| Density | 1.595 g/cm3 |
| Melting point | 563.7 °C; 1,046.7 °F; 836.9 K |
| Boiling point | 1,496 °C; 2,725 °F; 1,769 K |
| Solubility in water | 48 g/100 mL (10 °C) 82 g/100 mL (34.7 °C) |
| Refractive index (nD) | 1.452 |
| Hazards | |
| MSDS | ICSC 1118 |
| EU Index | 006-007-00-5 |
| EU classification | |
| R-phrases | R26/27/28, R32, R50/53 |
| S-phrases | (S1/2), S7, S28, S29, S45, S60, S61 |
| NFPA 704 |
 0
4
0
|
| Flash point | Non-flammable |
| LD50 | 5.8–15 mg/kg (oral in rats, mice)[2] |
| Related compounds | |
| Other cations | Potassium cyanide |
| Related compounds | Hydrogen cyanide |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Sodium cyanide is an inorganic compound with the formula NaCN. It is a white, water-soluble solid. Cyanide has a high affinity for metals, which leads to the high toxicity of this salt. Its main application, in gold mining, also exploits its high reactivity toward metals. When it is treated with acid, it forms the toxic gas hydrogen cyanide.
Production and chemical properties
Sodium cyanide is produced by treating hydrogen cyanide with sodium hydroxide:[3]
- HCN + NaOH → NaCN + H2O
Worldwide production was estimated at 500,000 tons in the year 2006. In former times, it was prepared by the Castner-Kellner process involving the reaction of sodium amide with carbon at elevated temperatures.
- NaNH2 + C → NaCN + H2
The structure of solid NaCN is related to that of sodium chloride.[4] The anions and cations are each six-coordinate. Potassium cyanide (KCN) adopts a similar structure. Each Na+ forms pi-bonds to two CN− groups as well as two "bent" Na---CN and two "bent" Na---NC links.[5]
Because the salt is derived from a weak acid, NaCN readily reverts to HCN by hydrolysis: the moist solid emits small amounts of hydrogen cyanide, which smells like bitter almonds (not everyone can smell it—the ability thereof is due to a genetic trait[6]). Sodium cyanide reacts rapidly with strong acids to release hydrogen cyanide. This dangerous process represents a significant risk associated with cyanide salts. It is detoxified most efficiently with hydrogen peroxide (H2O2) to produce sodium cyanate (NaOCN) and water:[3]
- NaCN + H2O2 → NaOCN + H2O
Applications
Cyanide mining
Sodium gold cyanide
Sodium cyanide is mainly used to extract gold and other precious metals in mining. This application exploits the high affinity of gold(I) for cyanide, which induces gold metal to oxidize and dissolve in the presence of air and water, producing the salt sodium gold cyanide (or gold sodium cyanide) and sodium hydroxide:
- 4 Au + 8 NaCN + O2 + 2 H2O → 4 Na[Au(CN)2] + 4 NaOH
A similar process uses potassium cyanide (KCN, a close relative of sodium cyanide) to produce potassium gold cyanide (KAu(CN)2).
Few other methods exist for this extraction process.
Chemical feedstock
Several commercially significant chemical compounds are derived from cyanide, including cyanuric chloride, cyanogen chloride, and many nitriles. In organic synthesis, cyanide, which is classified as a strong nucleophile, is used to prepare nitriles, which occur widely in many specialty chemicals, including pharmaceuticals.
Niche uses
Being highly toxic, sodium cyanide is used to kill or stun rapidly such as in widely illegal cyanide fishing and in collecting jars used by entomologists.
Toxicity
Cyanide salts are among the most rapidly acting of all known poisons. Cyanide is a potent inhibitor of respiration, acting on mitochondrial cytochrome oxidase and hence blocking electron transport. This results in decreased oxidative metabolism and oxygen utilization. Lactic acidosis then occurs as a consequence of anaerobic metabolism.
See also
References
- ↑ Oxford MSDS
- ↑ Martel, B.; Cassidy, K. (2004). Chemical Risk Analysis: A Practical Handbook. Butterworth–Heinemann. p. 361. ISBN 1-903996-65-1.
- ↑ 3.0 3.1 Andreas Rubo, Raf Kellens, Jay Reddy, Norbert Steier, Wolfgang Hasenpusch "Alkali Metal Cyanides" in Ullmann's Encyclopedia of Industrial Chemistry 2006 Wiley-VCH, Weinheim, Germany. doi:10.1002/14356007.i01_i01
- ↑ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ↑ H. T. Stokes, D. L. Decker, H. M. Nelson, J. D. Jorgensen (1993). "Structure of potassium cyanide at low temperature and high pressure determined by neutron diffraction". Phys. Rev. B 47 (17): 11082–11092. doi:10.1103/PhysRevB.47.11082.
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) 304300
External links
- Institut national de recherche et de sécurité (1997). "Cyanure de sodium. Cyanure de potassium". Fiche toxicologique n° 111, Paris:INRS, 6pp. (PDF file, in French)
- International Chemical Safety Card 1118
- Hydrogen cyanide and cyanides (CICAD 61)
- National Pollutant Inventory - Cyanide compounds fact sheet
- NIOSH Pocket Guide to Chemical Hazards
- EINECS number 205-599-4
- CID 8929 from PubChem
- CSST (Canada)
- Sodium cyanide hazards to fish and other wildlife from gold
| ||||||||