Skutterudite
| Skutterudite | |
|---|---|
 Skutterudite from Bou Azzer, Morocco | |
| General | |
| Category | Arsenide mineral |
| Formula (repeating unit) | (Co,Ni,Fe)As3 |
| Strunz classification | 02.EC.05 |
| Crystal symmetry | Isometric 2/m 3 |
| Unit cell | a = 8.204 Å, Z=8 |
| Identification | |
| Color | Tin-white to silver-gray, tarnishes gray or iridescent; in polished section, gray, creamy or golden white |
| Crystal habit | Crystals are cubes, octahedra, dodecahedra, rarely prismatic; in skeletal growth forms, distorted aggregates; also massive, granular |
| Crystal system | Cubic - Diploidal |
| Twinning | On {112} as sixlings and complex shapes |
| Cleavage | Distinct on {001} and {111}; in traces on {011} |
| Fracture | Conchoidal to uneven |
| Mohs scale hardness | 5.5 - 6 |
| Luster | Metallic |
| Streak | Black |
| Diaphaneity | Opaque |
| Specific gravity | 6.5 |
| References | [1][2][3][4] |
Skutterudite is a cobalt arsenide mineral that has variable amounts of nickel and iron substituting for cobalt with a general formula: (Co,Ni,Fe)As3. Some references give the arsenic a variable formula subscript of 2-3. High nickel varieties are referred to as nickel-skutterudite, previously chloanthite. It is a hydrothermal ore mineral found in moderate to high temperature veins with other Ni-Co minerals. Associated minerals are arsenopyrite, native silver, erythrite, annabergite, nickeline, cobaltite, silver sulfosalts, native bismuth, calcite, siderite, barite and quartz.[1] It is mined as an ore of cobalt and nickel with a by-product of arsenic.
The crystal structure of this mineral has been found to have important technological uses for several compounds isostructural with the mineral.
The mineral has a bright metallic luster, and is tin white or light steel gray in color with a black streak. The specific gravity is 6.5 and the hardness is 5.5-6. Its crystal structure is isometric with cube and octahedron forms similar to that of pyrite. The arsenic content gives a garlic odor when heated or crushed.
It was discovered in Skuterud Mines, Modum, Buskerud, Norway, in 1845.[2] Smaltite is a synonym for the mineral. Notable occurrences include Cobalt, Ontario, Skuterud, Norway, and Franklin, New Jersey in the United States. The rare arsenide minerals are classified in Dana's sulfide mineral group, even though it contains no sulfur.
The skutterudite crystal structure
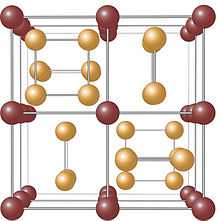
The crystal structure of the skutterudite mineral was determined in 1928 by Oftedahl to be cubic, belonging to space group Im-3 (number 204). The unit cell can be considered to consist of eight smaller cubes made up of the Co atoms. Six of these cubes are filled with (almost) square planar rings of As, each of which is oriented parallel to one of the unit cell edges, see image on the right. The As atoms then form octahedra with Co in the centre.
In crystallographic terms, the Co atoms occupy the 8c sites, while the As atoms occupy the 24g sites. The position of the Co atoms within the unit cell is fixed, while the positions of the As atoms are determined by the parameters x and y. It has been shown that for the As-rings to be fully square, these parameters must satisfy the Oftedahl relation x+y=1/2. Any deviation from this relation yields a rectangular configuration of the As atoms. Indeed, this is the case for all known compounds with this structure, and the As atoms then do not form perfect octahedra.
Together with the unit cell size and the assigned space group, these two parameters fully describe the crystal structure of the material which is often referred to as the skutterudite structure.
References
- ↑ 1.0 1.1 http://rruff.geo.arizona.edu/doclib/hom/skutterudite.pdf Handbook of Mineralogy
- ↑ 2.0 2.1 http://www.mindat.org/min-3682.html Mindat.org
- ↑ http://webmineral.com/data/Skutterudite.shtml Webmineral data
- ↑ Klein, Cornelis and Cornelius S. Hurlbut, jr., Manual of Mineralogy, Wiley, 20th ed., 1985, p. 289 ISBN 0-471-80580-7
- A. Kjekshus and T. Rakke. Compounds with skutterudite type crystal-structure .3. Structural data for arsenides and antimonides. Acta Chemica Scandinavica Series A 28 (1): 99-103 1974.