Siroheme
From Wikipedia, the free encyclopedia
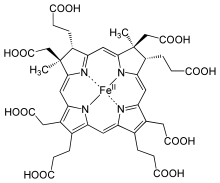
Structure of siroheme
Siroheme (or sirohaem) is a heme-like prosthetic group used by some enzymes to accomplish the six-electron reduction of sulfur and nitrogen.[1] Siroheme is synthesized from uroporphyrinogen III, a heme and vitamin B12 precursor.[2] It plays a major role in the sulfur assimilation pathway: converting sulfite to a biologically useful sulfide, which can be incorporated into the organic compound homocysteine.[3]
See also
- Ferredoxin-nitrite reductase
- Hydrogensulfite reductase
- Nitrite reductase (NAD(P)H)
References
- ↑ Matthew J. Murphy; et al. (1974). "Siroheme: A New Prosthetic Group Participating in Six-Electron Reduction Reactions Catalyzed by Both Sulfite and Nitrite Reductases". PNAS 71 (3): 612–616. doi:10.1073/pnas.71.3.612. PMC 388061. PMID 4595566.
- ↑ Jorgen Hansen; et al. (1997). "Siroheme biosynthesis in Saccharomyces cerevisiae requires the products of both MET1 and MET8 genes". FEBS Letters 401 (1): 20–24. doi:10.1016/S0014-5793(96)01423-8. PMID 9003798.
- ↑ Dominique Thomas; Yolande Surdin-Kerjan (1997). "Metabolism of sulfur amino acids in Saccharomyces cerevisiae". Microbiology and Molecular Biology Reviews 61 (4): 503–532. PMC 232622. PMID 9409150.
| ||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.