Single displacement reaction
A single-displacement reaction, also named single-replacement reaction, is a type of oxidation-reduction chemical reaction when an element or ion moves out of one compound and into another - that is, one element is replaced by another in a compound. This is represented by the general reaction scheme:
- A + BC → AC + B
This will most often occur if A is more reactive than B.
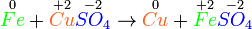 |
A and B must be either:
- Different metals (hydrogen's behavior as a cation renders it as a metal here), in which case C represents an anion; or
- Halogens, in which case C represents a cation.
In either case, when AC and BC are aqueous compounds (which is usually the case), C is a spectator ion.

In the activity or reactivity series, the metals with the highest propensity to donate their electrons to react are listed first, and the most unreactive metals are listed last. Therefore a metal higher on the list is able to displace anything on the list below it.[1] The order of activity for metals, from most reactive to least reactive, is Li, K, Sr, Ca, Na, Mg, Al, Zn, Cr, Fe, Cd, Co, Ni, Sn, Pb, Hg, Sb, As, Bi, Cu, Hg, Ag, Pd, Pt, Au. Similarly, the halogens with the highest propensity to acquire electrons are the most reactive. The activity series for halogens, from highest to lowest, is F, Cl, Br, I.[2]
Due to the free state nature of A and B, all single displacement reactions are also oxidation-reduction reactions, where the key event is the movement of electrons from one reactant to another. [3] When A and B are metals, A is always oxidized and B is always reduced. Since halogens prefer to gain electrons, A is reduced (from a 0 to −1) and B is oxidized (from −1 to 0) when A and B represent those elements.
A and B may have different charge as ions and therefore some balancing of the equation may be necessary. For example the reaction between silver nitrate, AgNO3 (which contains an Ag+ ion), and zinc, Zn, forms silver, Ag, and zinc nitrate, Zn(NO3)2 (which contains a Zn2+ ion).
- 2AgNO3(aq) + Zn(s) → 2Ag(s) + Zn(NO3)2(aq)
All simple metal with acid reactions are single displacement reactions. For example the reaction between magnesium, Mg, and hydrochloric acid, HCl, forms magnesium chloride, MgCl2, and hydrogen, H2.
- Mg(s) + 2 HCl(aq) → MgCl2(aq) + H2(g)
Cation replacement
One cation replaces another. A cation is a positively charged ion or a metal. When it is written in generic symbols, it is written out like this:
- AX + Y → YX + A
Element Y has replaced A (in a compound AX) to become a new compound YX and the free element A. This is an oxidation-reduction reaction wherein element A is reduced from a cation into the elemental form and element Y is oxidized from the elemental form into a cation. Some examples are:
- Cu + 2AgNO3 → 2Ag + Cu(NO3)2
- Fe + Cu(NO3)2 → Fe(NO3)2 + Cu
- Ca + 2H2O → Ca(OH)2 + H2
- Zn + 2HCl → ZnCl2 + H2
Note that if the reactant in elemental form is not the more reactive metal, then no reaction will occur. Some examples of this would be the reverse reactions to these:
- Ag + Cu(NO3)2 → No reaction
- Au + HCl → No reaction
Anion replacement
One anion replaces another. An anion is a negatively charged ion or a nonmetal. Written using generic symbol, it is:
- A + XY → XA + Y
Element A has replaced Y (in the compound XY) to form a new compound XA and the free element Y. This is an oxidation-reduction reaction wherein element A is reduced from the elemental form into an anion and element Y is oxidized from an anion into the elemental form.
Some of the few examples that involve halogens are shown here:
- Cl2 + 2NaBr → 2NaCl + Br2
- Br2 + 2KI → 2KBr + I2
Again, the less reactive halogen cannot replace the more reactive halogen:
- I2 + 2KBr → no reaction
See also
- Double displacement reaction
- Substitution reaction
References
- ↑ Barke, Hans-Dieter; Hazari, Al; Yitbarek, Sileshi (2008). Misconceptions in chemistry addressing perceptions in chemical education (Online-Ausg. ed.). Berlin: Springer. pp. 227–228. ISBN 3540709894.
- ↑ Brown, LeMay, Burston. Chemistry the Central Science, 10th ed. p. 143 Pearson Prentice Hall 2006
- ↑ Silberberg. Chemistry, the Molecular Nature of Matter and Change, 4th ed. p. 150 McGraw Hill 2006.