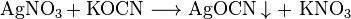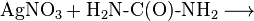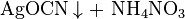Silver cyanate
From Wikipedia, the free encyclopedia
| Silver cyanate | ||
|---|---|---|
| Systematic name Silver(I) cyanate | ||
| Identifiers | ||
| CAS number | 3315-16-0 | |
| PubChem | 76827 | |
| ChemSpider | 69282 | |
| Jmol-3D images | {{#if:[Ag+].[O-]C#N|Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | AgOCN | |
| Molar mass | 149.885 g/mol | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Silver cyanate is a chemical compound and the cyanate salt of silver.
It can be prepared by reaction of potassium cyanate or urea with silver nitrate.[1]
Silver cyanate is a beige to gray powder. It crystallises in the monoclinic crystal system in space group P21/m with parameters a = 547.3 pm, b = 637.2 pm, c = 341.6 pm und β = 91°.[2]
With nitric acid silver cyanate reacts to form carbon dioxide and ammonium nitrate.[3]
See also
- Justus Von Liebig
- Friedrich Woehler
- Silver fulminate
- Isomerism
References
- ↑ Willy Kühne (1868) (in German), [Seite 470, p. 470, at Google Books Lehrbuch der physiologischen Chemie], Seite 470, p. 470, at Google Books
- ↑ D. Britton, J. D. Dunitz: The crystal structure of silver cyanate, Acta Cryst. (1965). 18, 424-428, doi:10.1107/S0365110X65000944
- ↑ J. Milbauer: Bestimmung und Trennung der Cyanate, Cyanide, Rhodanide und Sulfide in Fresenius' Journal of Analytical Chemistry 42 (1903) 77-95, doi:10.1007/BF01302741.
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.