Secular equilibrium
In nuclear physics, secular equilibrium is a situation in which the quantity of a radioactive isotope remains constant because its production rate (e.g., due to decay of a parent isotope) is equal to its decay rate.
Secular equilibrium in radioactive decay
Secular equilibrium can only occur in a radioactive decay chain if the half-life of the daughter radionuclide B is much shorter than the half-life of the parent radionuclide A. In such a situation, the decay rate of A, and hence the production rate of B, is approximately constant, because the half-life of A is very long compared to the timescales being considered. The quantity of radionuclide B builds up until the number of B atoms decaying per unit time becomes equal to the number being produced per unit time; the quantity of radionuclide B then reaches a constant, equilibrium value. Assuming the initial concentration of radionuclide B is zero, full equilibrium usually takes several half-lives of radionuclide B to establish.
The quantity of radionuclide B when secular equilibrium is reached is determined by the quantity of its parent A and the half-lives of the two radionuclide. This can be seen from the time rate of change of the number of atoms of radionuclide B:
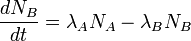 ,
,
where λA and λB are the decay constants of radionuclide A and B, related to their half-lives t1/2 by 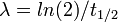 , and NA and NB are the number of atoms of A and B at a given time.
, and NA and NB are the number of atoms of A and B at a given time.
Secular equilibrium occurs when 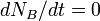 , or
, or
 .
.
Over long enough times, comparable to the half-life of radionuclide A, the secular equilibrium is only approximate; NA decays away according to
 ,
,
and the "equilibrium" quantity of radionuclide B declines in turn. For times short compared to the half-life of A,  and the exponential can be approximated as 1.
and the exponential can be approximated as 1.