Schiff base

-skeletal.png)
A Schiff base, named after Hugo Schiff, is a compound with a functional group that contains a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group, not hydrogen.[1] Schiff bases in a broad sense have the general formula R1R2C=NR3, where R is an organic side chain. In this definition, Schiff base is synonymous with azomethine. Some restrict the term to the secondary aldimines (azomethines where the carbon is connected to a hydrogen atom), thus with the general formula RCH=NR'.[2]
The chain on the nitrogen makes the Schiff base a stable imine. A Schiff base derived from an aniline, where R3 is a phenyl or a substituted phenyl, can be called an anil.[3]
Synthesis
Schiff bases can be synthesized from an aliphatic or aromatic amine and a carbonyl compound by nucleophilic addition forming a hemiaminal, followed by a dehydration to generate an imine. In a typical reaction, 4,4'-diaminodiphenyl ether reacts with o-vanillin:[4]
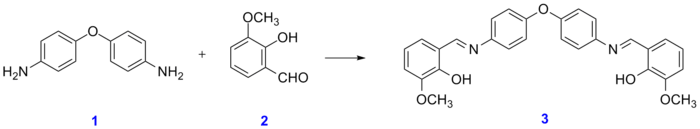
Biochemistry
Schiff bases are common enzymatic intermediates where an amine, such as the terminal group of a lysine residue reversibly reacts with an aldehyde or ketone of a cofactor or substrate. The common enzyme cofactor PLP forms a Schiff base with a lysine residue and is transaldiminated to the substrate(s).[5] Similarly, the cofactor retinal forms a Schiff base in rhodopsins, including human rhodopsin (via Lysine 296), which is key in the photoreception mechanism.
An example where the substrate forms a Schiff base to the enzyme is in the fructose 1,6-bisphosphate aldolase catalyzed reaction during glycolysis and in the metabolism of amino acids.
Coordination chemistry
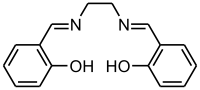
Schiff bases are common ligands in coordination chemistry. The imine nitrogen is basic and exhibits pi-acceptor properties. The ligands are typically derived from alkyl diamines and aromatic aldehydes.[6]
| Schiff base ligands | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
Chiral Schiff bases were one of the first ligands used for asymmetric catalysis. In 1968 Ryōji Noyori developed a copper-Schiff base complex for the metal-carbenoid cyclopropanation of styrene.[7] For this work he was later awarded a share of the 2001 Nobel Prize in Chemistry.

References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Schiff base".
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "azomethines".
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "anils".
- ↑ Jarrahpour, A. A.; M. Zarei (February 24, 2004). "Synthesis of 2-({[4-(4-{[(E)-1-(2-hydroxy-3-methoxyphenyl)methylidene amino}phenoxy)phenyl imino}methyl)- 6 -methoxy phenol". Molbank M352. ISSN 1422-8599. Retrieved February 22, 2010.
- ↑ Eliot, A. C.; Kirsch, J. F. (2004). "PYRIDOXALPHOSPHATEENZYMES: Mechanistic, Structural, and Evolutionary Considerations". Annual Review of Biochemistry 73: 383–415. doi:10.1146/annurev.biochem.73.011303.074021. PMID 15189147.
- ↑ R. Hernández-Molina, A. Mederos "Acyclic and Macrocyclic Schiff Base Ligands" in Comprehensive Coordination Chemistry II 2003, Pages 411–446. doi:10.1016/B0-08-043748-6/01070-7
- ↑ H. Nozaki, H. Takaya, S. Moriuti, R. Noyori (1968). "Homogeneous catalysis in the decomposition of diazo compounds by copper chelates: Asymmetric carbenoid reactions". Tetrahedron 24 (9): 3655–3669. doi:10.1016/S0040-4020(01)91998-2.
2.png)
.png)