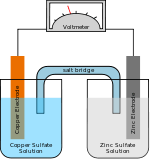
Salt bridge

A salt bridge, in electrochemistry, is a laboratory device used to connect the oxidation and reduction half-cells of a galvanic cell (voltaic cell), a type of electrochemical cell. Salt bridges usually come in two types: glass tube and filter paper.
Glass tube bridges
One type of salt bridge consists of a U-shaped glass tube filled with a relatively inert electrolyte; usually potassium chloride or sodium chloride is used, although the diagram here illustrates the use of a potassium nitrate solution. The electrolyte is so chosen that
- it does not react with any of the chemicals used in the cell, and
- the anion and cation have similar conductivity, and hence similar migratory speed.
The electrolyte is often gelified with agar- agar to help prevent the intermixing of fluids which might otherwise occur.
The conductivity of a glass tube bridge depends mostly on the concentration of the electrolyte solution. An increase in concentration below saturation increases conductivity. Beyond-saturation electrolyte content and narrow tube diameter may both lower conductivity.
Filter paper bridges
The other type of salt bridge consists of a filter paper, also soaked with a relatively inert electrolyte, usually potassium chloride or sodium chloride because they are chemically inert. No gelification agent is required as the filter paper provides a solid medium for conduction.
Conductivity of this kind of salt bridge depends on a number of factors: the concentration of the electrolyte solution, the texture of the filter paper and the absorbing ability of the filter paper. Generally, smoother texture and higher absorbency equates to higher conductivity.
A porous disk or other porous barrier between the two half-cells may be used instead of a salt bridge; however, they basically serve the same purpose.
Uses
As electrons leave one half of a galvanic cell and flow to the other, a difference in charge is built up. If no salt bridge were used, this increasing charge difference would eventually prevent further flow of electrons. A salt bridge allows the flow of ions to maintain a balance in charge between the oxidation and reduction vessels while keeping the contents of each separate. With the charge difference balanced, electrons can flow once again, and the reduction and oxidation reactions can proceed. In general, keeping the two cells separate is preferable from the point of view of eliminating variables from an experiment. When no direct contact between electrolytes is allowed, there is no need to make allowance for possible interactions between ionic species.
The technique more specifically allows freedom in the choice of ions in solution. For instance, a mixture of two different cations in solution might result in the preferential reduction of the wrong one, for the purposes of the experiment. With a salt bridge, the desired cation (positive) species is isolated in one vessel while the cation in the other vessel may be chosen to make the experiment easier, such as using a more soluble, or more stable salt of the anionic (negative) species.
If the two vessels are entirely disconnected, without a salt bridge, then the cation and anion species are also isolated, but then each species quickly reaches equilibrium because no electrical current can flow. The salt bridge can be seen as a way of completing the ionic circuit without letting the solutions intermix.