Ruthenocene
| Ruthenocene | ||
|---|---|---|
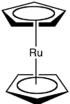 | ||
 |
 | |
| IUPAC name Ruthenocene | ||
| Other names ruthenium cyclopentadienyl, cp2Ru | ||
| Identifiers | ||
| CAS number | 1287-13-4 | |
| PubChem | 11986121 | |
| Jmol-3D images | {{#if:[cH-]1cccc1.[cH-]1cccc1.[Ru+2]|Image 1 | |
| ||
| Properties | ||
| Molecular formula | C10H10Ru | |
| Molar mass | 231.26 g/mol | |
| Appearance | pale yellow powder | |
| Density | 1.86 g/cm3 (25 °C) | |
| Melting point | 195-200 °C | |
| Boiling point | 278 °C | |
| Solubility in water | Insoluble in water, soluble in most organic solvents | |
| Hazards | ||
| EU classification | | |
| R-phrases | R36/37/38 | |
| S-phrases | S26, S28, S37/39, S45 | |
| NFPA 704 |
 0
2
0
| |
| Related compounds | ||
| Related compounds | cobaltocene, nickelocene, chromacene, ferrocene, bis(benzene)chromium | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Ruthenocene is an organoruthenium compound with the formula (C5H5)2Ru. This pale yellow, volatile solid is classified as a sandwich compound and more specifically, as a metallocene.
Structure and bonding
Ruthenocene consists of a ruthenium ion sandwiched in between two cyclopentadienyl rings. It features ruthenium centre bound symmetrically to the planes of two cyclopentadienyl rings. It is closely related to the isoelectronic ferrocene.
In contrast to ferrocene, wherein the cyclopentadienyl rings are in a staggered conformation, those of ruthenocene crystallise with an eclipsed conformation. This difference is due to the larger ionic radius of ruthenium, which increases the distance between the cyclopentadienyl rings, decreasing steric interactions and allowing an eclipsed conformation to prevail. In solution, these rings rotate with a very low barrier.
Preparation
Ruthenocene was first synthesized in 1952 by Geoffrey Wilkinson, a Nobel laureate who had collaborated in assigning the structure of ferrocene only a year earlier.[1] Originally, ruthenocene was prepared by the reaction of ruthenium trisacetylacetonate with excess of cyclopentadienylmagnesium bromide.[1]
- Ru(acac)3 + 3 C5H5MgBr → Ru(C5H5)2 + 3 "acacMgBr" + "C5H5"
Ruthenocene may also be prepared by the reaction of sodium cyclopentadienide with "ruthenium dichloride" (prepared from ruthenium metal and ruthenium trichloride in situ).[2]
Chemical properties
Ruthenocene typically oxidises via two electron change, instead of one.[3] With weakly coordinating anions as electrolyte, the oxidation proceeds via a 1e step.[4]
Ruthenocene has been investigated as a photoinitiator for polymerization reactions.[5]
References
- ↑ 1.0 1.1 Wilkinson, G. (1952). "The Preparation and Some Properties of Ruthenocene and Ruthenicinium Salts". J. Am. Chem. Soc. 74 (23): 6146. doi:10.1021/ja01143a538..
- ↑ Bublitz, D. E; McEwen, W. E.; Kleinberg, J. (1973), "Ruthenocene", Org. Synth.; Coll. Vol. 5: 1001
- ↑ Smith, T. P; Taube, H.; Bino, A.; Cohen, S. (1984). "Reactivity of Haloruthenocene(IV) complexes". Inorg. Chem. 23 (13): 1943. doi:10.1021/ic00181a030.
- ↑ Geiger, W. E. and Barrière, F., "Organometallic Electrochemistry Based on Electrolytes Containing Weakly-Coordinating Fluoroarylborate Anions", Accounts of Chemical Research, 2010. doi:10.1021/ar1000023.
- ↑ Cynthia T. Sanderson, Bentley J. Palmer, Alan Morgan, Michael Murphy, Richard A. Dluhy, Todd Mize, I. Jonathan Amster, and Charles Kutal "Classical Metallocenes as Photoinitiators for the Anionic Polymerization of an Alkyl 2-Cyanoacrylate" Macromolecules 2002, volume 35, pp. 9648-9652.doi:10.1021/ma0212238
| ||||||||||||||||||||||||||