Ruthenium(III) acetylacetonate
| Ruthenium(III) acetylacetonate | ||
|---|---|---|
_acac.png) | ||
| IUPAC name Tris(acetylacetonato)Ruthenium (III) | ||
| Other names Ru(acac)3; Ruthenium (III) 2,4-Pentanedionate; Ruthenium (III) acetylacetonato, 2,4-pentanedione ruthenium (III) | ||
| Identifiers | ||
| CAS number | 14284-93-6 | |
| PubChem | 16057901 | |
| Jmol-3D images | {{#if:CC(=O)CC(=O)C.CC(=O)CC(=O)C.CC(=O)CC(=O)C.[Ru]|Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | (C5H7O2)3Ru | |
| Molar mass | 398.39 g/mol | |
| Appearance | Dark Violet Solid | |
| Density | 1.54 g/cm3[1] | |
| Melting point | 260 °C | |
| Solubility in water | insoluble in water | |
| Solubility | soluble in most organic solvents | |
| Hazards | ||
| R-phrases | R36, R37, R38 | |
| S-phrases | S26, S36 | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Ruthenium(III) acetylacetonate is a coordination complex with the formula Ru(O2C5H7)3. O2C5H7)3 is the ligand called acetylacetonate. This compound exists as a dark violet solid that is soluble in most organic solvents.[2] It is used as a precursor to other compounds of ruthenium.
Preparation
In 1914 tris(acetylacetonato)ruthenium (III) was first prepared by the reaction of ruthenium(III) chloride and acetylacetone in the presence of potassium bicarbonate.[3] Since then, alternative synthetic routes have been examined, but the original procedure remains useful with minor variations:[4]
- RuCl3•3H2O + MeCOCH2COMe → Ru(acac)3 + 3 HCl + 3 H2O
Structure and properties
This compound has idealized D3 symmetry. Six oxygen atoms surround the central ruthenium atom in an octahedral arrangement. The average Ru-O bond length in Ru(acac)3 is 2.00 Å.[1] Because Ru(acac)3 is low spin, there is one unpaired d electron, causing this compound to be paramagnetic. Ru(acac)3 has a magnetic susceptibility, χM, of 3.032×10−6 cm3/mol with an effective magnetic moment, μeff, of 1.66 μB.[5] As a solution in DMF, the compound oxidizes at 0.593 and reduces at -1.223 V vs the ferrocene/ferrocenium couple.[6]
Reduction of Ru(acac)3 in the presence of alkenes affords the related diolefin complexes. Typically, such reactions are conducted with zinc amalgam in moist tetrahydrofuran:[7]
- 2 Ru(acac)3 + 4 alkene + Zn → 2 Ru(acac)2(alkene)2 + Zn(acac)2
The resulting compounds are rare examples of metal-alkene complexes that reversibly sustain oxidation:
- Ru(acac)2(alkene)2
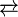 [Ru(acac)2(alkene)2]+ + e-
[Ru(acac)2(alkene)2]+ + e-
References
- ↑ 1.0 1.1 Chao, G.; Sime, R. L.; Sime, R. J. (1973). "Crystal and molecular structure of tris(acetylacetonato)ruthenium(III)". Acta Crystallographica B 29 (12): 2845. doi:10.1107/S0567740873007636.
- ↑ R.C. Mehrotra, R. Bohra, and D.P. Gaur "Metal β-Diketonates and Allied Derivatives", 1st ed.; Academic Press inc.: New York, 1978. ISBN 0-12-488150-5.
- ↑ Barbieri, G. A. (1914). "Systematic chemical investigations: ruthenium, rhodium, palladium". Atti accad, Lincei 23 (1): 334–40.
- ↑ Gupta, A. (2000). "Improved synthesis and reactivity of tris(acetylacetonato)ruthenium(III)". Indian journal of chemistry. Section A, Inorganic, bio-inorganic, physical, theoretical & analytical chemistry 39A (4): 457. ISSN 0376-4710.
- ↑ Grobelny, R. (1966). "The absorption spectra and magnetic properties of the chelated compounds of Ru(III) with β-diketones". Journal of inorganic and nuclear chemistry 28 (11): 2715. doi:10.1016/0022-1902(66)80398-6.
- ↑ Paul Sharpe, N. George Alameddin, David E. Richardson (1994). "Alkyl Substituent Effects in the Redox Thermochemistry of Coordination Compounds: Oxidation and Reduction Energetics for Ruthenium Tris(β-diketonate) Complexes in Solution and the Gas Phase". Journal of the American Chemical Society 116 (24): 11098–11108. doi:10.1021/ja00103a027.
- ↑ Bennett, M. A.; Byrnes, Matthew J.; Kováčik, Ivan (2004). "The fragment bis(acetylacetonato)ruthenium: a meeting-point of coordination and organometallic chemistry". Journal of Organometallic Chemistry 689 (24): 4463. doi:10.1016/j.jorganchem.2004.07.027.