Rhomboid protease
| Rhomboid | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Rhomboid | ||||||||
| Pfam | PF01694 | ||||||||
| Pfam clan | CL0207 | ||||||||
| InterPro | IPR002610 | ||||||||
| MEROPS | S54 | ||||||||
| SCOP | 144092 | ||||||||
| SUPERFAMILY | 144092 | ||||||||
| OPM superfamily | 186 | ||||||||
| OPM protein | 2ic8 | ||||||||
| |||||||||
The rhomboid proteases are a family of enzymes that exist in almost all species. They are proteases: they cut the polypeptide chain of other proteins. This proteolytic cleavage is irreversible in cells, and an important type of cellular regulation. Although proteases are one of the earliest and best studied class of enzyme, rhomboids belong to a much more recently discovered type: the intramembrane proteases. What is unique about intramembrane proteases is that their active sites are buried in the lipid bilayer of cell membranes, and they cleave other transmembrane proteins within their transmembrane domains.[1] About 30% of all proteins have transmembrane domains, and their regulated processing often has major biological consequences. Accordingly, rhomboids regulate many important cellular processes, and may be involved in a wide range of human diseases.
Intramembrane proteases
Rhomboids are intramembrane serine proteases.[2][3][4] The other types of intramembrane protease are aspartyl- and metallo-proteases, respectively. The presenilins and signal peptide peptidase-like family, which are intramembrane aspartyl proteases, cleave substrates that include the Notch receptor and the amyloid precursor protein, which is implicated in Alzheimer's disease. The site-2 protease family, which are intramembrane metalloproteases, regulate among other things cholesterol biosynthesis and stress responses in bacteria. The different intramembrane protease families are evolutionarily and mechanistically unrelated, but there are clear common functional themes that link them. Rhomboids are perhaps the best characterised class.
History of rhomboid discovery
Rhomboids were first named after a mutation in the fruitfly Drosophila, discovered in a famous genetic screen that led to a Nobel Prize for Christiane Nüsslein-Volhard and Eric Wieschaus.[5] In that screen they found a number of mutants with similar phenotypes: ‘pointy’ embryonic head skeletons. They named them each with a pointy-themed name – one was rhomboid. Genetic analysis later proved that this group of genes were members of the epidermal growth factor (EGF) receptor signalling pathway,[6][7] and that rhomboid was needed to generate the signal that activates the EGF receptor.[8][9] The molecular function of rhomboid took a bit longer to unravel but a combination of genetics and molecular techniques led to the discovery that Drosophila rhomboid and other members of the family were the first known intramembrane serine proteases.[2]
Biological role of rhomboids
Rhomboids were first discovered as proteases that regulate EGF receptor signalling in Drosophila. By releasing the extracellular domain of the growth factor Spitz, from its transmembrane precursor, rhomboid triggers signalling.[2][10] Since then, many other important biological functions have been proposed.[11][12]
- Although less well established than in Drosophila, there is some evidence that rhomboids may participate in growth factor signalling in mammals, including humans.[13] They have also been implicated in ephrin signalling [14] and the cleavage of the anticoagulant protein thrombomodulin.[15]
- All eukaryotes have a mitochondrial rhomboid. In yeast this has been shown to control mitochondrial function and morphology by regulating membrane fusion via the cleavage of a dynamin-like GTPase called Mgm1p, the orthologue of human OPA1.[16][17] In Drosophila, the mitochondrial rhomboid also regulates mitochondrial membrane fusion.[18] In mammals too, mitochondrial function is disrupted in mutants of PARL, the mitochondrial rhomboid, but the range of functions is more complex. PARL regulates the remodelling of mitochondrial cristae,[19] is implicated in cell death [19] and metabolism,[20] and there is increasing evidence of an important role in Parkinson's Disease;[21][22][23]
- Apicomplexan parasites like Plasmodium (the agent that causes malaria) and Toxoplasma appear to use rhomboids to cleave cell surface proteins that participate in the host invasion process;[24][25][26][27] they have also been implicated in the pathogenicity of other parasites;[28]
- A rhomboid in the Gram-negative bacterium Providencia stuartii is required for the function of the twin-arginine protein translocation (TAT) machinery.[29]
Structure and enzyme function of rhomboids
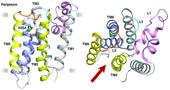
Rhomboids were the first intramembrane proteases for which a high resolution crystal structure was solved.[30][31][32][33][34] These structures confirmed predictions that rhomboids have a core six transmembrane domain structure, and that the catalytic site depends on a serine and histidine catalytic dyad. The structure also explained how a proteolytic reaction, which requires water molecules, can occur in the hydrophobic environment of a lipid bilayer: one of the central mysteries of intramembrane proteases.[35] The catalytic site was shown to be a hydrophilic indentation, protected from the lipid bilayer by surrounding transmembrane domains.[30][31][32][33][34]
One area of uncertainty is the route by which substrates get access to the rhomboid active site . Although substrates were initially proposed to enter between transmembrane domains 1 and 3,[30][33] evidence now strongly supports an alternative access point, between transmembrane domains 2 and 5.[31][32][34][36][37]
Rhomboid specificity
Rhomboids do not cleave all transmembrane domains. In fact, they are highly specific, with a limited number of substrates. Most natural Rhomboid substrates known so far are type 1 single transmembrane proteins, with their amino termini in the luminal/extracellular compartment. However, recent studies suggested that type 2 membrane protein (i.e. with opposite topology: the amino terminus is cytoplasmic), or even multipass membrane proteins could act as rhomboid substrates.[38] The specificity of rhomboids underlies their ability to control functions in a wide range of biological processes and, in turn, understanding what makes a particular transmembrane domain into a rhomboid substrate can shed light on rhomboid function in different contexts.
Initial work indicated that rhomboids recognise instability of the transmembrane alpha-helix as the main substrate determinant.[24][39] More recently a primary sequence motif in or immediately adjacent to the transmembrane domain has been shown to be the cardinal recognition determinant of a variety of rhomboid substrates.[40] This recognition motif directs where the substrate is cleaved. This can occur either within the transmembrane domain or just outside the membrane. Only in the former case are helix destabilising residues also necessary. Although the structure of a rhomboid/substrate complex has not yet been solved, a recent structure of the enzyme in complex with a mechanism-based inhibitor [41] is consistent with the current understanding of rhomboid specificity.[42]
In some Gram-negative bacteria, including Shewanella and Vibrio, up to thirteen proteins proteins are found with GlyGly-CTERM, a C-terminal homology domain consisting of a glycine-rich motif, a highly hydrophobic transmembrane helix, and a cluster of basic residues. This domain appears to be the recognition sequence for rhombosortase, a branch of the rhomboid protease family limited to just those bacteria with the GlyGly-CTERM domain.[43]
Medical significance of rhomboids
The diversity of biological functions already known to depend on rhomboids is reflected in evidence that rhomboids play a role in a variety if diseases including cancer, parasite infection, and diabetes.[11][12] It is important to note, however, that there is no case yet established where a precise medical significance is fully validated.
No drugs that modulate rhomboid activity have yet been reported, although a recent study has identified small molecule, mechanism-based inhibitors that could provide a basis for future drug development.[44]
The rhomboid-like family
Rhomboid proteases appear to be conserved in all eukaryotes and the vast majority of prokaryotes. Bioinformatic analysis highlights that some members of the rhomboid family lack the amino acid residues essential for proteolysis, implying that they cannot cleave substrates. These ‘pseudoproteases’ include a subfamily that have been named the iRhoms [45] (also known as RHBDF1 and RHBDF2). iRhoms can promote the ER associated degradation (ERAD) of EGF receptor ligands.[46] In Drosophila this provides a mechanism for regulating EGF receptor activity in the brain. This unexpected mechanism implies that the fundamental cellular quality control mechanism is exploited by multicellular organisms to regulate signalling between cells.
Phylogenetic analysis indicates that rhomboids are in fact members of a larger rhomboid-like superfamily or clan, which includes the derlin proteins, also involved in ERAD.[47]
References
- ↑ Brown, M. S., Ye, J., Rawson, R. B. & Goldstein, J. L. Cell 100, 391-38. (2000)
- ↑ 2.0 2.1 2.2 S. Urban, J. R. Lee, M. Freeman, Cell 107, 173 (2001)
- ↑ Lemberg, M. K. et al. EMBO J. 24, 464-472 (2005
- ↑ Urban, S. & Wolfe, M. S. Proc. Natl. Acad. Sci. U S A 102, 1883-1888 (2005)
- ↑ G. Jürgens, E. Wieschaus, C. Nüsslein-Volhard, H. Kluding, Wilhelm Roux’s Arch. Dev. Biol. 193, 283 (1984)
- ↑ M. A. Sturtevant, M. Roark, E. Bier, Genes Dev. 7, 961 (1993)
- ↑ M. Freeman, Mech Dev 48, 25 (1994)
- ↑ J. D. Wasserman, S. Urban, M. Freeman, Genes Dev. 14, 1651 (2000)
- ↑ A. G. Bang, C. Kintner, Genes Dev. 14, 177 (2000)
- ↑ J. R. Lee, S. Urban, C. F. Garvey, M. Freeman, Cell 107, 161 (2001)
- ↑ 11.0 11.1 M. Freeman, Annu. Rev. Genet. 42, 191 (2008)
- ↑ 12.0 12.1 S. Urban, Nat Rev Microbiol (2009)
- ↑ C. Adrain, K. Strisovsky, M. Zettl, L. Hu, M. Lemberg, M. Freeman, EMBO Rep. 12, 421 (2011)
- ↑ J. C. Pascall, K. D. Brown, Biochem. Biophys. Res. Commun. 317, 244 (2004)
- ↑ O. Lohi, S. Urban, M. Freeman, Curr. Biol. 14, 236 (2004)
- ↑ M. Herlan, F. Vogel, C. Bornhovd, W. Neupert, A. S. Reichert, J. Biol. Chem. 278, 27781 (2003)
- ↑ G. A. McQuibban, S. Saurya, M. Freeman, Nature 423, 537 (2003)
- ↑ G. A. McQuibban, J. R. Lee, L. Zheng, M. Juusola, M. Freeman, Curr. Biol. 16, 982 (2006)
- ↑ 19.0 19.1 S. Cipolat et al., Cell 126, 163 (2006)
- ↑ A. E. Civitarese et al., Cell Metab 11, 412 (2010).
- ↑ A. J. Whitworth et al., Dis Model Mech 1, 168 (2008)
- ↑ E. Deas et al., Hum. Mol. Genet. 20, 867 (2011)
- ↑ C. Meissner, H. Lorenz, A. Weihofen, D. J. Selkoe, M. K. Lemberg, J. Neurochem. 117, 856 (2011)
- ↑ 24.0 24.1 S. Urban, M. Freeman, Mol. Cell. 11, 1425 (2003)
- ↑ R. P. Baker, R. Wijetilaka, S. Urban, PLoS Pathog. 2, e113 (2006)
- ↑ R. A. O'Donnell et al., J. Cell Biol. 174, 1023 (2006)
- ↑ J. M. Santos, D. J. Ferguson, M. J. Blackman, D. Soldati-Favre, Science 331, 473 (2011)
- ↑ L. A. Baxt, R. P. Baker, U. Singh, S. Urban, Genes Dev. 22, 1636 (2008)
- ↑ L. G. Stevenson, K. Strisovsky, K. M. Clemmer, S. Bhatt, M. Freeman, P. N. Rather, Proc. Natl. Acad. Sci. U S A 104, 1003 (2007)
- ↑ 30.0 30.1 30.2 Y. Wang, Y. Zhang, Y. Ha, Nature 444, 179 (2006)
- ↑ 31.0 31.1 31.2 Z. Wu et al., Nat Struct Mol Biol 13, 1084 (2006)
- ↑ 32.0 32.1 32.2 A. Ben-Shem, D. Fass, E. Bibi, Proc. Natl. Acad. Sci. U S A 104, 462 (2007)
- ↑ 33.0 33.1 33.2 M. J. Lemieux, S. J. Fischer, M. M. Cherney, K. S. Bateman, M. N. James, Proc. Natl. Acad. Sci. U S A 104, 750 (2007).
- ↑ 34.0 34.1 34.2 K. R. Vinothkumar, J. Mol. Biol. 407, 232 (2011)
- ↑ M. K. Lemberg, M. Freeman, Mol. Cell. 28, 930 (2007)
- ↑ R. P. Baker, K. Young, L. Feng, Y. Shi, S. Urban, Proc. Natl. Acad. Sci. U S A 104, 8257 (2007)
- ↑ Y. Wang, S. Maegawa, Y. Akiyama, Y. Ha, J. Mol. Biol. 374, 1104 (2007)
- ↑ R. Tsruya et al., EMBO J. 26, 1211 (2007)
- ↑ Y. Akiyama, S. Maegawa, Mol. Microbiol. 64, 1028 (2007)
- ↑ K. Strisovsky, H. J. Sharpe, M. Freeman, Mol. Cell. 36, 1048 (2009)
- ↑ K. R. Vinothkumar, K. Strisovsky, A. Andreeva, Y. Christova, S. Verhelst, M. Freeman, EMBO J. 29, 3797 (2010)
- ↑ K. Strisovsky, FEBS J. 280, 1579-1603 (2013)
- ↑ D. H. Haft and N. Varghese, PLoS One 6, e28886 (2011)
- ↑ O. Pierrat, K. Strisovsky et al., ACS Chem Biol (2010)
- ↑ M. K. Lemberg, M. Freeman, Genome Res. 17, 1634 (2007)
- ↑ M. Zettl, C. Adrain, K. Strisovsky, V. Lastun, M. Freeman, Cell 145, 79 (2011)
- ↑ http://pfam.sanger.ac.uk/clan/rhomboid-like
External links