Rhodium(IV) oxide
From Wikipedia, the free encyclopedia
| Rhodium(IV) oxide | |
|---|---|
 | |
| Identifiers | |
| CAS number | 12137-27-8 |
| PubChem | 82936 |
| ChemSpider | 15017693 |
| EC number | 235-237-0 |
| Jmol-3D images | {{#if:[Rh+4].[O-2].[O-2]|Image 1 |
| |
| |
| Properties | |
| Molecular formula | RhO2 |
| Molar mass | 134.904 g/mol |
| Appearance | black crystalline solid |
| Density | 7.2 g/cm³ |
| Melting point | 1050 °C (decomposes) |
| Solubility | insoluble in aqua regia |
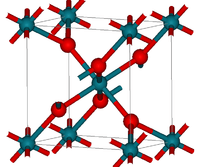
| Structure | |
| Crystal structure | tetragonal (rutile) |
| Hazards | |
| EU classification | not listed |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Rhodium(IV) oxide (or Rhodium dioxide) is the chemical compound with the formula RhO2.
Chemical properties
RhO2 is highly insoluble even in hot aqua regia.[1]
Structure
RhO2 has the tetragonal rutile structure.[2]
Physical properties
RhO2 has metallic resistivity with values <10−4 Ohm·cm. It transforms in air to RhO3 at 850 °C and then to metal and oxygen at 1050 °C.[2]
See also
References
- ↑ O. Muller and R. Roy (1968). "Formation and stability of the platinum and rhodium oxides at high oxygen pressures and the structures of Pt3O4, β-PtO2 and RhO2". Journal of the Less Common Metals 16: 129–146. doi:10.1016/0022-5088(68)90070-2.
- ↑ 2.0 2.1 R. D. Shannon (1968). "Synthesis and properties of two new members of the rutile family RhO2 and PtO2". Solid State Communications 6: 139–143. doi:10.1016/0038-1098(68)90019-7.
| |||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.