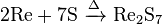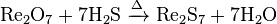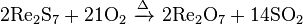Rhenium(VII) sulfide
From Wikipedia, the free encyclopedia
| Rhenium(VII) sulfide | ||
|---|---|---|
| IUPAC name Rhenium(VII) sulfide | ||
| Identifiers | ||
| CAS number | 12038-67-4 | |
| ChemSpider | 21171359 | |
| Jmol-3D images | {{#if:S=[Re](=S)(=S)S[Re](=S)(=S)=S|Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | Re2S7 | |
| Molar mass | 596.869 g/mol | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Rhenium(VII) sulfide is a chemical compound with the formula Re2S7. It can be produced through the reaction of ReO4- and H2S in 4N HCl.[1]
Synthesis
- Direct combination of rhenium and sulfur:
- Treating rhenium(VII) oxide with hydrogen sulfide:
Reactions
Rhenium(VII) sulfide decomposes when heated in vacuum:
It is converted to oxide when heated in air:
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0080379419.
- Химическая энциклопедия / Редкол.: Кнунянц И.Л. и др.. — М.: Советская энциклопедия, 1995. — Т. 4. — 639 с. — ISBN 5-82270-092-4 (Russian)
- Справочник химика / Редкол.: Никольский Б.П. и др.. — 3-е изд., испр. — Л.: Химия, 1971. — Т. 2. — 1168 с. (Russian)
- Рипан Р., Четяну И. Неорганическая химия. Химия металлов. — М.: Мир, 1972. — Т. 2. — 871 с. (Russian)
| ||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is available under the Creative Commons Attribution/Share Alike; additional terms may apply for the media files.