Restriction enzyme
The cutting of DNA at specific boundaries
Used by restriction enzymes to locate specific sequences of DNA on which to bind and subsequently cleave
The DNA sequence to which restriction enzymes bind
The DNA sequence that is cleaved by the restriction enzyme
A restriction enzyme (or restriction endonuclease) is an enzyme that cuts DNA at or near specific recognition nucleotide sequences known as restriction sites.[1][2][3] Restriction enzymes are commonly classified into three types, which differ in their structure and whether they cut their DNA substrate at their recognition site, or if the recognition and cleavage sites are separate from one another. To cut DNA, all restriction enzymes make two incisions, once through each sugar-phosphate backbone (i.e. each strand) of the DNA double helix.
These enzymes are found in bacteria and archaea and provide a defense mechanism against invading viruses.[4][5] Inside a prokaryote, the restriction enzymes selectively cut up foreign DNA in a process called restriction; while host DNA is protected by a modification enzyme (a methylase) that modifies the prokaryotic DNA and blocks cleavage. Together, these two processes form the restriction modification system.[6]
Over 3000 restriction enzymes have been studied in detail, and more than 600 of these are available commercially.[7] These enzymes are routinely used for DNA modification in laboratories, and are a vital tool in molecular cloning.[8][9][10]
History
The term restriction enzyme originated from the studies of phage λ and the phenomenon of host-controlled restriction and modification of a bacterial virus.[11] The phenomenon was first identified in work done in the laboratories of Salvador Luria and Giuseppe Bertani in early 1950s.[12][13] It was found that a bacteriophage λ that can grow well in one strain of Escherichia coli, for example E. coli C, when grown in another strain, for example E. coli K, its yields can drop significantly, by as much as 3-5 orders of magnitude. The E. coli K host cell, known as the restricting host, appears to have the ability to reduce the biological activity of the phage λ. If a phage becomes established in one strain, the ability of that phage to grow also become restricted in other strains. In the 1960s, it was shown in work done in the laboratories of Werner Arber and Matthew Meselson that the restriction is caused by an enzymatic cleavage of the phage DNA, and the enzyme involved was therefore termed a restriction enzyme.[4][14][15][16]
The restriction enzymes studied by Arber and Meselson were type I restriction enzymes which cleave DNA randomly away from the recognition site.[17] In 1970, Hamilton O. Smith, Thomas Kelly and Kent Welcox isolated and characterized the first type II restriction enzyme, HindII, from the bacterium Haemophilus influenzae.[18][19] This type of restriction enzymes is more useful for laboratory use as they cleave DNA at the site of their recognition sequence. It was later shown by Daniel Nathans and Kathleen Danna that cleavage of simian virus 40 (SV40) DNA by restriction enzymes yielded specific fragments which can be separated using polyacrylamide gel electrophoresis, thus showing that restriction enzymes can be used for mapping of the DNA.[20] For their work in the discovery and characterization of restriction enzymes, the 1978 Nobel Prize for Physiology or Medicine was awarded to Werner Arber, Daniel Nathans, and Hamilton O. Smith.[21] Their discovery led to the development of recombinant DNA technology that allowed, for example, the large scale production of human insulin for diabetics using E. coli bacteria.[22][12]
Origins
Restriction enzymes likely evolved from a common ancestor and became widespread via horizontal gene transfer. [23][24] In addition, there is mounting evidence that restriction endonucleases evolved as a selfish genetic element. [25]
Recognition site

Restriction enzymes recognize a specific sequence of nucleotides[2] and produce a double-stranded cut in the DNA. The recognition sequences usually vary between 4 and 8 nucleotides, and many of them are palindromic, meaning the base sequence reads the same backwards and forwards.[26] In theory, there are two types of palindromic sequences that can be possible in DNA. The mirror-like palindrome is similar to those found in ordinary text, in which a sequence reads the same forward and backwards on a single strand of DNA strand, as in GTAATG. The inverted repeat palindrome is also a sequence that reads the same forward and backwards, but the forward and backward sequences are found in complementary DNA strands (i.e., of double-stranded DNA), as in GTATAC (GTATAC being complementary to CATATG).[27] Inverted repeat palindromes are more common and have greater biological importance than mirror-like palindromes.
EcoRI digestion produces "sticky" ends,
![]()
whereas SmaI restriction enzyme cleavage produces "blunt" ends:
![]()
Recognition sequences in DNA differ for each restriction enzyme, producing differences in the length, sequence and strand orientation (5' end or the 3' end) of a sticky-end "overhang" of an enzyme restriction.[28]
Different restriction enzymes that recognize the same sequence are known as neoschizomers. These often cleave in different locales of the sequence. Different enzymes that recognize and cleave in the same location are known as isoschizomers.
Types
Naturally occurring restriction endonucleases are categorized into four groups (Types I, II III, and IV) based on their composition and enzyme cofactor requirements, the nature of their target sequence, and the position of their DNA cleavage site relative to the target sequence.[29][30][31] All types of enzymes recognize specific short DNA sequences and carry out the endonucleolytic cleavage of DNA to give specific fragments with terminal 5'-phosphates. They differ in their recognition sequence, subunit composition, cleavage position, and cofactor requirements,[32][33] as summarised below:
- Type I enzymes (EC 3.1.21.3) cleave at sites remote from recognition site; require both ATP and S-adenosyl-L-methionine to function; multifunctional protein with both restriction and methylase (EC 2.1.1.72) activities.
- Type II enzymes (EC 3.1.21.4) cleave within or at short specific distances from recognition site; most require magnesium; single function (restriction) enzymes independent of methylase.
- Type III enzymes (EC 3.1.21.5) cleave at sites a short distance from recognition site; require ATP (but do not hydrolyse it); S-adenosyl-L-methionine stimulates reaction but is not required; exist as part of a complex with a modification methylase (EC 2.1.1.72).
- Type IV enzymes target modified DNA, e.g. methylated, hydroxymethylated and glucosyl-hydroxymethylated DNA
Type I
Type I restriction enzymes were the first to be identified and were first identified in two different strains (K-12 and B) of E. coli.[34] These enzymes cut at a site that differs, and is a random distance (at least 1000 bp) away, from their recognition site. Cleavage at these random sites follows a process of DNA translocation, which shows that these enzymes are also molecular motors. The recognition site is asymmetrical and is composed of two specific portions—one containing 3–4 nucleotides, and another containing 4–5 nucleotides—separated by a non-specific spacer of about 6–8 nucleotides. These enzymes are multifunctional and are capable of both restriction and modification activities, depending upon the methylation status of the target DNA. The cofactors S-Adenosyl methionine (AdoMet), hydrolyzed adenosine triphosphate (ATP), and magnesium (Mg2+) ions, are required for their full activity. Type I restriction enzymes possess three subunits called HsdR, HsdM, and HsdS; HsdR is required for restriction; HsdM is necessary for adding methyl groups to host DNA (methyltransferase activity) and HsdS is important for specificity of the recognition (DNA-binding) site in addition to both restriction (DNA cleavage) and modification (DNA methyltransferase) activity.[29][34]
Type II
| Type II site-specific deoxyribonuclease | |||||||||
|---|---|---|---|---|---|---|---|---|---|
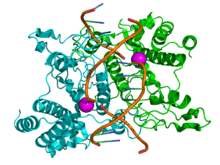 Structure of the homodimeric restriction enzyme EcoRI (cyan and green cartoon diagram) bound to double stranded DNA (brown tubes).[35] Two catalytic magnesium ions (one from each monomer) are shown as magenta spheres and are adjacent to the cleaved sites in the DNA made by the enzyme (depicted as gaps in the DNA backbone). | |||||||||
| Identifiers | |||||||||
| EC number | 3.1.21.4 | ||||||||
| CAS number | 9075-08-5 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
Typical type II restriction enzymes differ from type I restriction enzymes in several ways. They are a homodimer, with recognition sites are usually undivided and palindromic and 4–8 nucleotides in length. They recognize and cleave DNA at the same site, and they do not use ATP or AdoMet for their activity—they usually require only Mg2+ as a cofactor.[26] These are the most commonly available and used restriction enzymes. In the 1990s and early 2000s, new enzymes from this family were discovered that did not follow all the classical criteria of this enzyme class, and new subfamily nomenclature was developed to divide this large family into subcategories based on deviations from typical characteristics of type II enzymes.[26] These subgroups are defined using a letter suffix.
Type IIB restriction enzymes (e.g. BcgI and BplI) are multimers, containing more than one subunit.[26] They cleave DNA on both sides of their recognition to cut out the recognition site. They require both AdoMet and Mg2+ cofactors. Type IIE restriction endonucleases (e.g. NaeI) cleave DNA following interaction with two copies of their recognition sequence.[26] One recognition site acts as the target for cleavage, while the other acts as an allosteric effector that speeds up or improves the efficiency of enzyme cleavage. Similar to type IIE enzymes, type IIF restriction endonucleases (e.g. NgoMIV) interact with two copies of their recognition sequence but cleave both sequences at the same time.[26] Type IIG restriction endonucleases (Eco57I) do have a single subunit, like classical Type II restriction enzymes, but require the cofactor AdoMet to be active.[26] Type IIM restriction endonucleases, such as DpnI, are able to recognize and cut methylated DNA.[26] Type IIS restriction endonucleases (e.g. FokI) cleave DNA at a defined distance from their non-palindromic asymmetric recognition sites.[26] These enzymes may function as dimers. Similarly, Type IIT restriction enzymes (e.g., Bpu10I and BslI) are composed of two different subunits. Some recognize palindromic sequences while others have asymmetric recognition sites.[26]
Type III
Type III restriction enzymes (e.g. EcoP15) recognize two separate non-palindromic sequences that are inversely oriented. They cut DNA about 20-30 base pairs after the recognition site.[36] These enzymes contain more than one subunit and require AdoMet and ATP cofactors for their roles in DNA methylation and restriction, respectively.[37] They are components of prokaryotic DNA restriction-modification mechanisms that protect the organism against invading foreign DNA. Type III enzymes are hetero-oligomeric, multifunctional proteins composed of two subunits, Res and Mod. The Mod subunit recognises the DNA sequence specific for the system and is a modification methyltransferase; as such it is functionally equivalent to the M and S subunits of type I restriction endonuclease. Res is required for restriction, although it has no enzymatic activity on its own. Type III enzymes recognise short 5-6 bp long asymmetric DNA sequences and cleave 25-27 bp downstream to leave short, single-stranded 5' protrusions. They require the presence of two inversely oriented unmethylated recognition sites for restriction to occur. These enzymes methylate only one strand of the DNA, at the N-6 position of adenosyl residues, so newly replicated DNA will have only one strand methylated, which is sufficient to protect against restriction. Type III enzymes belong to the beta-subfamily of N6 adenine methyltransferases, containing the nine motifs that characterise this family, including motif I, the AdoMet binding pocket (FXGXG), and motif IV, the catalytic region (S/D/N (PP) Y/F).[38][39]
Type IV
Type IV enzymes recognize modified, typically methylated DNA and are exemplified by the McrBC and Mrr systems of E. coli.[40]
Type V
Type V restriction enzymes (e.g., the cas9-gRNA complex from CRISPRs[41]) utilize guide RNAs to target specific non-palindromic sequences found on invading organisms. They can cut DNA of variable length provided that a suitable guide RNA is provided. The flexibility and ease of use of these enzymes make them promising for future genetic engineering applications.[42][41][43]
Artificial restriction enzymes
Artificial restriction enzymes can be generated by fusing a natural or engineered DNA binding domain to a nuclease domain (often the cleavage domain of the type IIS restriction enzyme FokI[44]). Such artificial restriction enzymes can target large DNA sites (up to 36 bp) and can be engineered to bind to desired DNA sequences.[45] Zinc finger nucleases are the most commonly used artificial restriction enzymes and are generally used in genetic engineering applications,[46][47][48][49] but can also be used for more standard gene cloning applications.[50] Other artificial restriction enzymes are based on the DNA binding domain of TAL effectors.[51][52]
Nomenclature
| Derivation of the EcoRI name | ||
|---|---|---|
| Abbreviation | Meaning | Description |
| E | Escherichia | genus |
| co | coli | species |
| R | RY13 | strain |
| I | First identified | order of identification in the bacterium |
Since their discovery in the 1970s, more than 100 different restriction enzymes have been identified in different bacteria. Each enzyme is named after the bacterium from which it was isolated using a naming system based on bacterial genus, species and strain.[53][54] For example, the name of the EcoRI restriction enzyme was derived as shown in the box.
Applications
- See the main article on restriction digests.
Isolated restriction enzymes are used to manipulate DNA for different scientific applications.
They are used to assist insertion of genes into plasmid vectors during gene cloning and protein expression experiments. For optimal use, plasmids that are commonly used for gene cloning are modified to include a short polylinker sequence (called the multiple cloning site, or MCS) rich in restriction enzyme recognition sequences. This allows flexibility when inserting gene fragments into the plasmid vector; restriction sites contained naturally within genes influence the choice of endonuclease for digesting the DNA since it is necessary to avoid restriction of wanted DNA while intentionally cutting the ends of the DNA. To clone a gene fragment into a vector, both plasmid DNA and gene insert are typically cut with the same restriction enzymes, and then glued together with the assistance of an enzyme known as a DNA ligase.[55][56]
Restriction enzymes can also be used to distinguish gene alleles by specifically recognizing single base changes in DNA known as single nucleotide polymorphisms (SNPs).[57][58] This is only possible if a SNP alters the restriction site present in the allele. In this method, the restriction enzyme can be used to genotype a DNA sample without the need for expensive gene sequencing. The sample is first digested with the restriction enzyme to generate DNA fragments, and then the different sized fragments separated by gel electrophoresis. In general, alleles with correct restriction sites will generate two visible bands of DNA on the gel, and those with altered restriction sites will not be cut and will generate only a single band. The number of bands reveals the sample subject's genotype, an example of restriction mapping.[citation needed]
In a similar manner, restriction enzymes are used to digest genomic DNA for gene analysis by Southern blot. This technique allows researchers to identify how many copies (or paralogues) of a gene are present in the genome of one individual, or how many gene mutations (polymorphisms) have occurred within a population. The latter example is called restriction fragment length polymorphism (RFLP).[59]
Examples
- See the main article on list of restriction enzyme cutting sites.
Examples of restriction enzymes include:[60]
| Enzyme | Source | Recognition Sequence | Cut |
|---|---|---|---|
| EcoRI | Escherichia coli |
5'GAATTC 3'CTTAAG |
5'---G AATTC---3' 3'---CTTAA G---5' |
| EcoRII | Escherichia coli |
5'CCWGG 3'GGWCC |
5'--- CCWGG---3' 3'---GGWCC ---5' |
| BamHI | Bacillus amyloliquefaciens |
5'GGATCC 3'CCTAGG |
5'---G GATCC---3' 3'---CCTAG G---5' |
| HindIII | Haemophilus influenzae |
5'AAGCTT 3'TTCGAA |
5'---A AGCTT---3' 3'---TTCGA A---5' |
| TaqI | Thermus aquaticus |
5'TCGA 3'AGCT |
5'---T CGA---3' 3'---AGC T---5' |
| NotI | Nocardia otitidis |
5'GCGGCCGC 3'CGCCGGCG |
5'---GC GGCCGC---3' 3'---CGCCGG CG---5' |
| HinfI | Haemophilus influenzae |
5'GANTCA 3'CTNAGT |
5'---G ANTC---3' 3'---CTNA G---5' |
| Sau3A | Staphylococcus aureus |
5'GATC 3'CTAG |
5'--- GATC---3' 3'---CTAG ---5' |
| PvuII* | Proteus vulgaris |
5'CAGCTG 3'GTCGAC |
5'---CAG CTG---3' 3'---GTC GAC---5' |
| SmaI* | Serratia marcescens |
5'CCCGGG 3'GGGCCC |
5'---CCC GGG---3' 3'---GGG CCC---5' |
| HaeIII* | Haemophilus aegyptius |
5'GGCC 3'CCGG |
5'---GG CC---3' 3'---CC GG---5' |
| HgaI[61] | Haemophilus gallinarum |
5'GACGC 3'CTGCG |
5'---NN NN---3' 3'---NN NN---5' |
| AluI* | Arthrobacter luteus |
5'AGCT 3'TCGA |
5'---AG CT---3' 3'---TC GA---5' |
| EcoRV* | Escherichia coli |
5'GATATC 3'CTATAG |
5'---GAT ATC---3' 3'---CTA TAG---5' |
| EcoP15I | Escherichia coli |
5'CAGCAGN25NN 3'GTCGTCN25NN |
5'---CAGCAGN25 NN---3' 3'---GTCGTCN25NN ---5' |
| KpnI[62] | Klebsiella pneumoniae |
5'GGTACC 3'CCATGG |
5'---GGTAC C---3' 3'---C CATGG---5' |
| PstI[62] | Providencia stuartii |
5'CTGCAG 3'GACGTC |
5'---CTGCA G---3' 3'---G ACGTC---5' |
| SacI[62] | Streptomyces achromogenes |
5'GAGCTC 3'CTCGAG |
5'---GAGCT C---3' 3'---C TCGAG---5' |
| SalI[62] | Streptomyces albus |
5'GTCGAC 3'CAGCTG |
5'---G TCGAC---3' 3'---CAGCT G---5' |
| ScaI*[62] | Streptomyces caespitosus |
5'AGTACT 3'TCATGA |
5'---AGT ACT---3' 3'---TCA TGA---5' |
| SpeI | Sphaerotilus natans |
5'ACTAGT 3'TGATCA |
5'---A CTAGT---3' 3'---TGATC A---5' |
| SphI[62] | Streptomyces phaeochromogenes |
5'GCATGC 3'CGTACG |
5'---GCATG C---3' 3'---C GTACG---5' |
| StuI*[63][64] | Streptomyces tubercidicus |
5'AGGCCT 3'TCCGGA |
5'---AGG CCT---3' 3'---TCC GGA---5' |
| XbaI[62] | Xanthomonas badrii |
5'TCTAGA 3'AGATCT |
5'---T CTAGA---3' 3'---AGATC T---5' |
Key:
* = blunt ends
N = C or G or T or A
W = A or T
See also
- Detailed articles about certain restriction enzymes: EcoRI, HindIII, BglII.
- Homing endonuclease
- Isoschizomer.
- List of homing endonuclease cutting sites
- List of restriction enzyme cutting sites
- Molecular weight size marker
- Star activity
References
- ↑ Roberts RJ (November 1976). "Restriction endonucleases". CRC Crit. Rev. Biochem. 4 (2): 123–64. doi:10.3109/10409237609105456. PMID 795607.
- ↑ 2.0 2.1 Kessler C, Manta V (August 1990). "Specificity of restriction endonucleases and DNA modification methyltransferases a review (Edition 3)". Gene 92 (1–2): 1–248. doi:10.1016/0378-1119(90)90486-B. PMID 2172084.
- ↑ Pingoud A, Alves J, Geiger R (1993). "Chapter 8: Restriction Enzymes". In Burrell M. Enzymes of Molecular Biology. Methods of Molecular Biology 16. Totowa, NJ: Humana Press. pp. 107–200. ISBN 0-89603-234-5.
- ↑ 4.0 4.1 Arber W, Linn S (1969). "DNA modification and restriction". Annu. Rev. Biochem. 38: 467–500. doi:10.1146/annurev.bi.38.070169.002343. PMID 4897066.
- ↑ Krüger DH, Bickle TA (September 1983). "Bacteriophage survival: multiple mechanisms for avoiding the deoxyribonucleic acid restriction systems of their hosts". Microbiol. Rev. 47 (3): 345–60. PMC 281580. PMID 6314109.
- ↑ Kobayashi I (September 2001). "Behavior of restriction–modification systems as selfish mobile elements and their impact on genome evolution". Nucleic Acids Res. 29 (18): 3742–56. doi:10.1093/nar/29.18.3742. PMC 55917. PMID 11557807.
- ↑ Roberts RJ, Vincze T, Posfai J, Macelis D. (2007). "REBASE—enzymes and genes for DNA restriction and modification". Nucleic Acids Res 35 (Database issue): D269–70. doi:10.1093/nar/gkl891. PMC 1899104. PMID 17202163.
- ↑ Primrose SB, Old RW (1994). Principles of gene manipulation: an introduction to genetic engineering. Oxford: Blackwell Scientific. ISBN 0-632-03712-1.
- ↑ Micklos DA, Bloom MV, Freyer GA (1996). Laboratory DNA science: an introduction to recombinant DNA techniques and methods of genome analysis. Menlo Park, Calif: Benjamin/Cummings Pub. Co. ISBN 0-8053-3040-2.
- ↑ Massey A, Kreuzer H (2001). Recombinant DNA and Biotechnology: A Guide for Students. Washington, D.C: ASM Press. ISBN 1-55581-176-0.
- ↑ Winnacker E-L (1987). "Chapter 2: Isolation, Identification, and Characterisation of DNA fragments". From Genes to Clones. VCH. ISBN 0-89573-614-4.
- ↑ 12.0 12.1 Luria SE, Human ML (October 1952). "A nonhereditary, host-induced variation of bacterial viruses". J. Bacteriol. 64 (4): 557–69. PMC 169391. PMID 12999684.
- ↑ Bertani G, Weigle JJ (February 1953). "Host controlled variation in bacterial viruses". J. Bacteriol. 65 (2): 113–21. PMC 169650. PMID 13034700.
- ↑ Meselson M, Yuan R (March 1968). "DNA restriction enzyme from E. coli". Nature 217 (5134): 1110–4. Bibcode:1968Natur.217.1110M. doi:10.1038/2171110a0. PMID 4868368.
- ↑ Dussoix D, Arber W (July 1962). "Host specificity of DNA produced by Escherichia coli. II. Control over acceptance of DNA from infecting phage lambda". J. Mol. Biol. 5 (1): 37–49. doi:10.1016/S0022-2836(62)80059-X. PMID 13888713.
- ↑ Lederberg S, Meselson M (May 1964). "Degradtion of Non-replicating Bacteriophage DNA In Non-accepting Cells". J. Mol. Biol. 8 (5): 623–8. doi:10.1016/S0022-2836(64)80112-1. PMID 14187389.
- ↑ Roberts RJ (April 2005). "How restriction enzymes became the workhorses of molecular biology". Proc. Natl. Acad. Sci. U.S.A. 102 (17): 5905–8. Bibcode:2005PNAS..102.5905R. doi:10.1073/pnas.0500923102. PMC 1087929. PMID 15840723.
- ↑ Smith HO, Wilcox KW (July 1970). "A restriction enzyme from Hemophilus influenzae. I. Purification and general properties". J. Mol. Biol. 51 (2): 379–91. doi:10.1016/0022-2836(70)90149-X. PMID 5312500.
- ↑ Kelly TJ, Smith HO (July 1970). "A restriction enzyme from Hemophilus influenzae. II". J. Mol. Biol. 51 (2): 393–409. doi:10.1016/0022-2836(70)90150-6. PMID 5312501.
- ↑ Danna K, Nathans D (December 1971). "Specific cleavage of simian virus 40 DNA by restriction endonuclease of Hemophilus influenzae". Proc. Natl. Acad. Sci. U.S.A. 68 (12): 2913–7. Bibcode:1971PNAS...68.2913D. doi:10.1073/pnas.68.12.2913. PMC 389558. PMID 4332003.
- ↑ "The Nobel Prize in Physiology or Medicine". The Nobel Foundation. 1978. Retrieved 2008-06-07. "for the discovery of restriction enzymes and their application to problems of molecular genetics"
- ↑ Villa-Komaroff L, Efstratiadis A, Broome S, Lomedico P, Tizard R, Naber SP, Chick WL, Gilbert W (August 1978). "A bacterial clone synthesizing proinsulin". Proc. Natl. Acad. Sci. U.S.A. 75 (8): 3727–31. Bibcode:1978PNAS...75.3727V. doi:10.1073/pnas.75.8.3727. PMC 392859. PMID 358198.
- ↑ Jeltsch A, Manfred K, Pingroud A (1995). "Evidence for an evolutionary relationship among type-II restriction endonucleases". Gene 160 (1): 7–16. doi:10.1016/0378-1119(95)00181-5. PMID 7628720.
- ↑ Jeltsch A, Pingroud A (1996). "Horizontal gene transfer contributes to the wide distribution and evolution of type II restriction-modification systems". J Mol Evol 42 (2): 91–6. doi:10.1007/BF02198833. PMID 8919860.
- ↑ Kobayashi (1995). "Selfish behavior of restriction-modification systems". Science 267 (5199): 897–9. Bibcode:1995Sci...267..897N. doi:10.1126/science.7846533. PMID 7846533. More than one of
|author=and|last=specified (help) - ↑ 26.0 26.1 26.2 26.3 26.4 26.5 26.6 26.7 26.8 26.9 Pingoud A, Jeltsch A (September 2001). "Structure and function of type II restriction endonucleases". Nucleic Acids Res. 29 (18): 3705–27. doi:10.1093/nar/29.18.3705. PMC 55916. PMID 11557805.
- ↑ Clark DP (2005). Molecular biology. Amsterdam: Elsevier Academic Press. ISBN 0-12-175551-7.
- ↑ Goodsell DS (2002). "The molecular perspective: restriction endonucleases". Stem Cells 20 (2): 190–1. doi:10.1634/stemcells.20-2-190. PMID 11897876.
- ↑ 29.0 29.1 Bickle TA, Krüger DH (June 1993). "Biology of DNA restriction". Microbiol. Rev. 57 (2): 434–50. PMC 372918. PMID 8336674.
- ↑ Boyer HW (1971). "DNA restriction and modification mechanisms in bacteria". Annu. Rev. Microbiol. 25: 153–76. doi:10.1146/annurev.mi.25.100171.001101. PMID 4949033.
- ↑ Yuan R (1981). "Structure and mechanism of multifunctional restriction endonucleases". Annu. Rev. Biochem. 50: 285–319. doi:10.1146/annurev.bi.50.070181.001441. PMID 6267988.
- ↑ Rao DN, Sistla S (2004). "S-Adenosyl-L-methionine-dependent restriction enzymes". Crit. Rev. Biochem. Mol. Biol. 39 (1): 1–19. doi:10.1080/10409230490440532. PMID 15121719.
- ↑ Williams RJ (2003). "Restriction endonucleases: classification, properties, and applications". Mol. Biotechnol. 23 (3): 225–43. doi:10.1385/MB:23:3:225. PMID 12665693.
- ↑ 34.0 34.1 Murray NE (June 2000). "Type I Restriction Systems: Sophisticated Molecular Machines (a Legacy of Bertani and Weigle)". Microbiol. Mol. Biol. Rev. 64 (2): 412–34. doi:10.1128/MMBR.64.2.412-434.2000. PMC 98998. PMID 10839821.
- ↑ PDB 1qps Gigorescu A, Morvath M, Wilkosz PA, Chandrasekhar K, Rosenberg JM (2004). "The integration of recognition and cleavage: X-ray structures of pre-transition state complex, post-reactive complex, and the DNA-free endonuclease". In Alfred M. Pingoud. Restriction Endonucleases (Nucleic Acids and Molecular Biology, Volume 14). Berlin: Springer. pp. 137–178. ISBN 3-540-20502-0.
- ↑ Dryden DT, Murray NE, Rao DN (September 2001). "Nucleoside triphosphate-dependent restriction enzymes". Nucleic Acids Res. 29 (18): 3728–41. doi:10.1093/nar/29.18.3728. PMC 55918. PMID 11557806.
- ↑ Meisel A, Bickle TA, Krüger DH, Schroeder C (January 1992). "Type III restriction enzymes need two inversely oriented recognition sites for DNA cleavage". Nature 355 (6359): 467–9. Bibcode:1992Natur.355..467M. doi:10.1038/355467a0. PMID 1734285.
- ↑ Sistla S, Rao DN (2004). "S-Adenosyl-L-methionine-dependent restriction enzymes". Crit. Rev. Biochem. Mol. Biol. 39 (1): 1–19. doi:10.1080/10409230490440532. PMID 15121719.
- ↑ Bourniquel AA, Bickle TA (November 2002). "Complex restriction enzymes: NTP-driven molecular motors". Biochimie 84 (11): 1047–59. doi:10.1016/S0300-9084(02)00020-2. PMID 12595133.
- ↑ https://www.neb.com/products/restriction-endonucleases/restriction-endonucleases/types-of-restriction-endonucleases
- ↑ 41.0 41.1 Rodolphe B. et al. (2007). "CRISPR provides acquired reistance against viruses in prokaryotes. 2007". Science 315 (5819): 1709–12. doi:10.1126/science.1138140. PMID 17379808.
- ↑ Philippe, H. and Rodolphe, B. CRISPR/Cas, the immune system of bacteria and archaea. Science 2010
- ↑ Le, C. et al. Multiplex genome engineering using CRISPR/Cas systems. 2013. Science
- ↑ Kim YG, Cha J, Chandrasegaran S (February 1996). "Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain". Proc. Natl. Acad. Sci. U.S.A. 93 (3): 1156–60. Bibcode:1996PNAS...93.1156K. doi:10.1073/pnas.93.3.1156. PMC 40048. PMID 8577732.
- ↑ Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD (September 2010). "Genome editing with engineered zinc finger nucleases". Nat. Rev. Genet. 11 (9): 636–46. doi:10.1038/nrg2842. PMID 20717154.
- ↑ Townsend JA, Wright DA, Winfrey RJ, Fu F, Maeder ML, Joung JK, Voytas DF (May 2009). "High frequency modification of plant genes using engineered zinc finger nucleases". Nature 459 (7245): 442–5. Bibcode:2009Natur.459..442T. doi:10.1038/nature07845. PMC 2743854. PMID 19404258.
- ↑ Shukla VK, Doyon Y, Miller JC, DeKelver RC, Moehle EA, Worden SE, Mitchell JC, Arnold NL, Gopalan S, Meng X, Choi VM, Rock JM, Wu YY, Katibah GE, Zhifang G, McCaskill D, Simpson MA, Blakeslee B, Greenwalt SA, Butler HJ, Hinkley SJ, Zhang L, Rebar EJ, Gregory PD, Urnov FD (May 2009). "Precise genome modification in the crop species Zea mays using zinc-finger nucleases". Nature 459 (7245): 437–41. Bibcode:2009Natur.459..437S. doi:10.1038/nature07992. PMID 19404259.
- ↑ Ekker SC (2008). "Zinc Finger–Based Knockout Punches for Zebrafish Genes". Zebrafish 5 (2): 121–3. doi:10.1089/zeb.2008.9988. PMC 2849655. PMID 18554175.
- ↑ Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Ménoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R (July 2009). "Knockout Rats Produced Using Designed Zinc Finger Nucleases". Science 325 (5939): 433. Bibcode:2009Sci...325..433G. doi:10.1126/science.1172447. PMC 2831805. PMID 19628861.
- ↑ Tovkach A, Zeevi V, Tzfira T (October 2010). "Expression, purification and characterization of cloning-grade zinc finger nuclease". J Biotechnol 151 (1): 1–8. doi:10.1016/j.jbiotec.2010.10.071. PMID 21029755.
- ↑ Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, Bogdanove AJ, Voytas DF (October 2010). "Targeting DNA Double-Strand Breaks with TAL Effector Nucleases". Genetics 186 (2): 757–61. doi:10.1534/genetics.110.120717. PMC 2942870. PMID 20660643.
- ↑ Li T, Huang S, Jiang WZ, Wright D, Spalding MH, Weeks DP, Yang B (August 2010). "TAL nucleases (TALNs): hybrid proteins composed of TAL effectors and FokI DNA-cleavage domain". Nucleic Acids Res 39 (1): 359–372. doi:10.1093/nar/gkq704. PMC 3017587. PMID 20699274.
- ↑ Smith HO, Nathans D (December 1973). "Letter: A suggested nomenclature for bacterial host modification and restriction systems and their enzymes". J. Mol. Biol. 81 (3): 419–23. doi:10.1016/0022-2836(73)90152-6. PMID 4588280.
- ↑ Roberts RJ, Belfort M, Bestor T, Bhagwat AS, Bickle TA, Bitinaite J, Blumenthal RM, Degtyarev SKh, Dryden DT, Dybvig K, Firman K, Gromova ES, Gumport RI, Halford SE, Hattman S, Heitman J, Hornby DP, Janulaitis A, Jeltsch A, Josephsen J, Kiss A, Klaenhammer TR, Kobayashi I, Kong H, Krüger DH, Lacks S, Marinus MG, Miyahara M, Morgan RD, Murray NE, Nagaraja V, Piekarowicz A, Pingoud A, Raleigh E, Rao DN, Reich N, Repin VE, Selker EU, Shaw PC, Stein DC, Stoddard BL, Szybalski W, Trautner TA, Van Etten JL, Vitor JM, Wilson GG, Xu SY (April 2003). "SURVEY AND SUMMARY: A nomenclature for restriction enzymes, DNA methyltransferases, homing endonucleases and their genes". Nucleic Acids Res. 31 (7): 1805–12. doi:10.1093/nar/gkg274. PMC 152790. PMID 12654995.
- ↑ Geerlof A. "Cloning using restriction enzymes". European Molecular Biology Laboratory - Hamburg. Retrieved 2008-06-07.
- ↑ Russell DW, Sambrook J (2001). Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory. ISBN 0-87969-576-5.
- ↑ Wolff JN, Gemmell NJ (February 2008). "Combining allele-specific fluorescent probes and restriction assay in real-time PCR to achieve SNP scoring beyond allele ratios of 1:1000". BioTechniques 44 (2): 193–4, 196, 199. doi:10.2144/000112719. PMID 18330346.
- ↑ Zhang R, Zhu Z, Zhu H, Nguyen T, Yao F, Xia K, Liang D, Liu C (July 2005). "SNP Cutter: a comprehensive tool for SNP PCR–RFLP assay design". Nucleic Acids Res. 33 (Web Server issue): W489–92. doi:10.1093/nar/gki358. PMC 1160119. PMID 15980518.
- ↑ Stryer L, Berg JM, Tymoczko JL (2002). Biochemistry (Fifth ed.). San Francisco: W.H. Freeman. p. 122. ISBN 0-7167-4684-0.
- ↑ Roberts RJ (January 1980). "Restriction and modification enzymes and their recognition sequences". Nucleic Acids Res. 8 (1): r63–r80. doi:10.1093/nar/8.1.197-d. PMC 327257. PMID 6243774.
- ↑ Roberts RJ (1988). "Restriction enzymes and their isoschizomers". Nucleic Acids Res. 16 Suppl (Suppl): r271–313. doi:10.1093/nar/16.suppl.r271. PMC 340913. PMID 2835753.
- ↑ 62.0 62.1 62.2 62.3 62.4 62.5 62.6 Krieger M, Scott MP, Matsudaira PT, Lodish HF, Darnell JE, Zipursky L, Kaiser C, Berk A (2004). Molecular Cell Biology (5th ed.). New York: W.H. Freeman and Company. ISBN 0-7167-4366-3.
- ↑ "Stu I from Streptomyces tubercidicus". Sigma-Aldrich. Retrieved 2008-06-07.
- ↑ Shimotsu H, Takahashi H, Saito H (November 1980). "A new site-specific endonuclease StuI from Streptomyces tubercidicus". Gene 11 (3–4): 219–25. doi:10.1016/0378-1119(80)90062-1. PMID 6260571.
External links
| Library resources about Restriction enzymes |
Visualizations:
- Computer-generated animation of a restriction enzyme locating a target sequence and cutting the DNA there
General Information:
- DNA Restriction Enzymes at the US National Library of Medicine Medical Subject Headings (MeSH)
- Firman K (2007-11-24). "Type I Restriction-Modification". University of Portsmouth. Retrieved 2008-06-06.
- Goodsell DS (2000-08-01). "Restriction Enzymes". Molecule of the Month. RCSB Protein Data Bank. Retrieved 2008-06-06.
- Simmer M, Secko D (2003-08-01). "Restriction Endonucleases: Molecular Scissors for Specifically Cutting DNA". The Science Creative Quarterly. Retrieved 2008-06-06.
Databases:
- Roberts RJ, Vincze T, Posfai, J, Macelis D. "REBASE". Retrieved 2008-06-06. "Restriction Enzyme Database"
Software:
- Bikandi J, San Millán R, Rementeria A, and Garaizar J. "Restriction enzyme digest of DNA". insilico.ehu.es. Retrieved 2008-06-06.
- Palmer M. "WatCut". University of Waterloo, Ontario, Canada. Retrieved 2008-06-06. "An on-line tool for restriction analysis, silent mutation scanning, SNP-RFLP analysis"
- Vincze,T, Posfai J, Roberts RJ. "NEBcutter V2.0". New England Biolabs Inc. Retrieved 2008-06-06. "Restriction enzyme finder"
- "Restriction enzyme digest of DNA software". BioPHP: PHP for Bioinformatics. Retrieved 2008-06-06. "Online tool, free source code"
- "pDRAW32". AcaClone software. Retrieved 2008-06-06. "Freeware DNA cloning, sequence analysis and plasmid/DNA plotting software"
| ||||||||||||||||||||||||||||||||||||||||||||