Resazurin
| Resazurin | |
|---|---|
 | |
| IUPAC name 7-hydroxy-10-oxidophenoxazin-10-ium-3-one | |
| Identifiers | |
| CAS number | 550-82-3 [62758-13-8] (sodium salt) |
| PubChem | 11077 |
| ChemSpider | 10606 |
| ChEMBL | CHEMBL1616414 |
| Jmol-3D images | {{#if:C1=CC2=C(C=C1O)OC3=CC(=O)C=CC3=[N+]2[O-]|Image 1 |
| |
| |
| Properties | |
| Molecular formula | C12H7NO4 |
| Molar mass | 229.19 g mol−1 |
| Solubility in water | soluble |
| Hazards | |
| R-phrases | R22 R36/37/38 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |

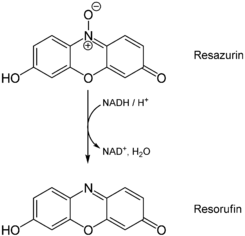
Resazurin (7-Hydroxy-3H-phenoxazin-3-one 10-oxide) is a blue dye, itself weakly fluorescent [1] until it is irreversibly reduced to the pink colored and highly red fluorescent resorufin. It is used as an oxidation-reduction indicator in cell viability assays for bacteria and mammalian cells, and for measuring aerobic respiration and exchange with the hyporheic zone in streams. Usually it is available commercially as the sodium salt.
| Resazurin (pH indicator) | ||
| below pH 3.8 | above pH 6.5 | |
| 3.8 | ↔ | 6.5 |
Resazurin solution has one of the highest values known of Kreft's dichromaticity index.[2] This means that it has a large change in perceived color hue when the thickness or concentration of observed sample increases or decreases.


Cell Viability applications
Resazurin was first used to quantify bacterial content in milk by Pesch and Simmert in 1929.[3] It is also used as an indicator for cell viability in mammalian cell cultures.[4] It was introduced commercially initially under Alamar Blue trademark (Trek Diagnostic Systems, Inc), and now also available under other names such as AB assay, Vybrant (Molecular Probes) and UptiBlue (Interchim).
Resazurin based assays show excellent correlation to reference viability assays such as formazan-based assays (MTT/XTT) and tritiated thymidine based techniques, while being much easier and safer to use for the user.[5] It also allows for longer studies (minimally toxic to living cells), works for adherents cells and bacteria/fungi.[5] Besides standard applications as cell culture assays (cell counting, cell proliferation assays[6]) and cytotoxicity testing, it also can be multiplexed with several chemiluminescent assays, such as cytokine assays, caspase assays to measure apoptosis, or reporter assays to measure a gene or a protein expression.[5] The irreversible reaction of resazurin to resorufin is proportional to aerobic respiration.[7]
Other applications
Resazurin is effectively reduced in mitochondria, making it useful also to assess mitochondrial metabolic activity.
Usually, in the presence of NADPH dehydrogenase or NADH dehydrogenase as the enzyme, NADPH or NADH is the reductant that converts resazurin to resorufin. Hence the resazurin/diaphorase/NADPH system can be used to detect NADH, NADPH, or diaphorase level, and any biochemical or enzyme activity that is involved in a biochemical reaction generating NADH or NADPH.[8][9][10][11][12]
Resazurin can be used to assay L-Glutamate, achieving a sensitivity of 2.0 pmol per well.[13]
Resazurin is used to measure the amount of aerobic respiration in streams[14] Since most aerobic respiration occurs in the stream bed, the conversion of resazurin to resorufin is also a measure of the amount of exchange between the water column and the stream bed.
Synthesis
Resazurin is prepared by acid-catalyzed condensation between resorcinol and 4-nitrosoresorcinol followed by oxidation of the intermediate with manganese(IV) oxide:

Treatment of the crude reaction product with excess sodium carbonate yields the sodium salt of resazurin, which is typically the commercial form of the dye. Running the condensation step in alcohols is possible but results in lower yields of the product; in pure water or acetic acid, the reaction does not proceed satisfactorily.[15]
Related dyes
10-acetyl-3,7-dihydroxyphenoxazine (also known as Amplex Red), structurally related to resazurin, reacts with H2O2 in a 1:1 stoichiometry to produce the same by-product resorufin (used in many assays combining for example horseradish peroxidase (HRP), or NADH, NADPH using enzymes).[16][17]
7-ethoxyresorufin, is a compound used as the substrate in the measurement of cytochrome P450 (CYP1A1) induction using the ethoxyresorufin-O-deethylase (EROD) assay system in cell culture and environmental samples, produced in response to exposure to aryl hydrocarbons. The compound is catalysed by the enzyme to produce the same fluorescent product, resorufin.[citation needed]
1,3-dichloro-7-hydroxy-9,9-dimethylacridin-2(9H)-one (DDAO dye), fluorescent dye used for oligonucleotide labeling.[citation needed]
References
- ↑ Bueno, C.; Villegas, M. L.; Bertolotti, S. G.; Previtali, C. M.; Neumann, M. G.; Encinas, M. V. (2002). "The Excited-State Interaction of Resazurin and Resorufin with Aminesin Aqueous Solutions. Photophysics and Photochemical Reaction". Photochemistry and Photobiology 76 (4): 385–90. doi:10.1562/0031-8655(2002)0760385TESIOR2.0.CO2. PMID 12405144.
- ↑ Kreft, Samo; Kreft, Marko (2009). "Quantification of dichromatism: A characteristic of color in transparent materials". Journal of the Optical Society of America A 26 (7): 1576–81. Bibcode:2009JOSAA..26.1576K. doi:10.1364/JOSAA.26.001576. PMID 19568292.
- ↑ Pesch, K. L.; Simmert, U. (1929). "Combined assays for lactose and galactose by enzymatic reactions". Milchw. Forsch 8: 551.
- ↑ Anoopkumar-Dukie, S; Carey, JB; Conere, T; O'Sullivan, E; Van Pelt, FN; Allshire, A (2005). "Resazurin assay of radiation response in cultured cells". British Journal of Radiology 78 (934): 945–7. doi:10.1259/bjr/54004230. PMID 16177019.
- ↑ 5.0 5.1 5.2 UptiBlue viable cell assay technical manual
- ↑ Kurin, Elena; Atanasov, Atanas; Donath, Oliver; Heiss, Elke; Dirsch, Verena; Nagy, Milan (2012). "Synergy Study of the Inhibitory Potential of Red Wine Polyphenols on Vascular Smooth Muscle Cell Proliferation". Planta Medica 78 (8): 772–8. doi:10.1055/s-0031-1298440. PMID 22499559.
- ↑ González-Pinzón, Ricardo; Haggerty, Roy; Myrold, David D. (2012). "Measuring aerobic respiration in stream ecosystems using the resazurin-resorufin system". Journal of Geophysical Research 117 (G3): G00N06. Bibcode:2012JGRG..117.0N06G. doi:10.1029/2012JG001965.
- ↑ Shahangian, S.; Ash, K. O.; Rollins, D. E. (1984). "An Enzymatic Method for the Analysis of Formate in Human Plasma". Journal of Analytical Toxicology 8 (6): 273–6. doi:10.1093/jat/8.6.273. PMID 6549198.
- ↑ Hanson, NQ; Freier, EF (1983). "Effect of protein on the determination of total bile acids in serum". Clinical Chemistry 29 (1): 171–5. PMID 6571720.
- ↑ De Jong, Donald W.; Woodlief, William G. (1977). "Fluorimetric assay of tobacco leaf dehydrogenases with resazurin". Biochimica et Biophysica Acta (BBA) - Enzymology 484 (2): 249–59. doi:10.1016/0005-2744(77)90081-X. PMID 20957.
- ↑ Barnes, Stephen; Spenney, Jerry G. (1980). "Stoichiometry of the nadh-oxidoreductase reaction for dehydrogenase determinations". Clinica Chimica Acta 107 (3): 149–54. doi:10.1016/0009-8981(80)90442-8. PMID 6893684.
- ↑ Winartasaputra, H; Mallet, VN; Kuan, SS; Guilbault, GG (1980). "Fluorometric and colorimetric enzymic determination of triglycerides (triacylglycerols) in serum". Clinical Chemistry 26 (5): 613–7. PMID 6894889.
- ↑ Chapman, Justin; Zhou, Mingjie (1999). "Microplate-based fluorometric methods for the enzymatic determination of l-glutamate: application in measuring l-glutamate in food samples". Analytica Chimica Acta 402 (1–2): 47–52. doi:10.1016/S0003-2670(99)00533-4.
- ↑ Haggerty, Roy; Martí, Eugènia; Argerich, Alba; Von Schiller, Daniel; Grimm, Nancy B. (2009). "Resazurin as a 'smart' tracer for quantifying metabolically active transient storage in stream ecosystems". Journal of Geophysical Research 114 (G3): G03014. Bibcode:2009JGRG..114.3014H. doi:10.1029/2008JG000942.
- ↑ A US 2376283 A, Frank Short Wallace & Peter Oxley, "Dyestuffs suitable for use as indicators", published 1945-05-15, assigned to Boots Pure Drug Co Ltd
- ↑ Zhou, M., Diwu, Z., Panchuk-Voloshina, N., et al. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: Applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem 253 162-168 (1997)
- ↑ https://www.caymanchem.com/app/template/Product.vm/catalog/10010469