Reaction rate constant
In chemical kinetics a reaction rate constant, k or 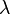 , quantifies the rate of a chemical reaction.[1]
, quantifies the rate of a chemical reaction.[1]
For a chemical reaction where substance A and B are reacting to produce C, the reaction rate has the form:
- Reaction: A + B → C
k(T) is the reaction rate constant that depends on temperature.
[A] is the concentration of substance A in moles per volume of solution assuming the reaction is taking place throughout the volume of the solution (for a reaction taking place at a boundary it would denote something like moles of A per area).
The exponents m and n are called partial orders and depend on the reaction mechanism. They can be determined experimentally.
A single-step reaction can also be written as
where Ea is the activation energy, and R is the gas constant. Since at temperature T the molecules have energies according to a Boltzmann distribution, one can expect the proportion of collisions with energy greater than Ea to vary with e−Ea/RT. A is the pre-exponential factor, or frequency factor.
The Arrhenius equation gives the quantitative basis of the relationship between the activation energy and the reaction rate at which a reaction proceeds.
Units
The units of the rate constant depend on the global order of reaction:[2] If concentration is measured in units of mol·L−1 (sometimes abbreviated as M), then
- For order (m + n), the rate coefficient has units of mol1−(m+n)·L(m+n)−1·s−1
- For order zero, the rate coefficient has units of mol·L−1·s−1 (or M·s−1)
- For order one, the rate coefficient has units of s−1
- For order two, the rate coefficient has units of L·mol−1·s−1 (or M−1·s−1)
- And for order three, the rate coefficient has units of L2·mol−2·s−1 (or M−2·s−1)
Plasma and gases
Calculation of rate constants of the processes of generation and relaxation of electronically and vibrationally excited particles are of significant importance. It is used, for example, in the computer simulation of processes in plasma chemistry or microelectronics. First-principle based models should be used for such calculation. It can be done with the help of computer simulation software.
See also
References
- ↑ http://www.chem.arizona.edu/~salzmanr/480a/480ants/chemkine.html
- ↑ Blauch, David. "Differential Rate Laws". Chemical Kinetics.
![r=k(T)[A]^{{m}}[B]^{{n}}](/2014-wikipedia_en_all_02_2014/I/media/2/d/a/2/2da2141720b7dcc6b8aa63c2b2af2112.png)
![r=Ae^{{\frac {-E_{a}}{RT}}}[A]^{m}[B]^{n},](/2014-wikipedia_en_all_02_2014/I/media/5/6/3/0/56305b547c63d525b479556748a9f93b.png)