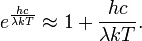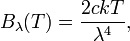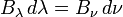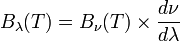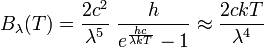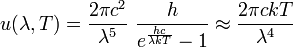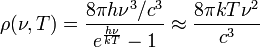Rayleigh–Jeans law

In physics, the Rayleigh–Jeans law attempts to describe the spectral radiance of electromagnetic radiation at all wavelengths from a black body at a given temperature through classical arguments. For wavelength λ, it is:
where c is the speed of light, k is the Boltzmann constant and T is the temperature in kelvins. For frequency ν, the expression is instead
The Rayleigh–Jeans law agrees with experimental results at large wavelengths (or, equivalently, low frequencies) but strongly disagrees at short wavelengths (or high frequencies). This inconsistency between observations and the predictions of classical physics is commonly known as the ultraviolet catastrophe,[1][2] and its resolution was a foundational aspect of the development of quantum mechanics in the early 20th century.
Historical development
In 1900, the British physicist Lord Rayleigh derived the λ−4 dependence of the Rayleigh–Jeans law based on classical physical arguments.[3] A more complete derivation, which included the proportionality constant, was presented by Rayleigh and Sir James Jeans in 1905. The Rayleigh–Jeans law revealed an important error in physics theory of the time. The law predicted an energy output that diverges towards infinity as wavelength approaches zero (as frequency tends to infinity) and measurements of energy output at short wavelengths disagreed with this prediction.
Comparison to Planck's law
In 1900 Max Planck empirically obtained an expression for black-body radiation expressed in terms of wavelength λ = c/ν (Planck's law):
where h is the Planck constant and k the Boltzmann constant. The Planck law does not suffer from an ultraviolet catastrophe, and agrees well with the experimental data, but its full significance (which ultimately led to quantum theory) was only appreciated several years later. Since,
then in the limit of very high temperatures or long wavelengths, the term in the exponential becomes small, and the exponential is well approximated with the Taylor polynomial's first-order term,
So,
This results in Planck's blackbody formula reducing to
which is identical to the classically derived Rayleigh–Jeans expression.
The same argument can be applied to the blackbody radiation expressed in terms of frequency ν = c/λ. In the limit of small frequencies, that is  ,
,
This last expression is the Rayleigh–Jeans law in the limit of small frequencies.
Consistency of frequency and wavelength dependent expressions
When comparing the frequency and wavelength dependent expressions of the Rayleigh–Jeans law it is important to remember that
because 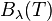 has units of energy emitted per unit time per unit area of emitting surface, per unit solid angle, per unit wavelength, whereas
has units of energy emitted per unit time per unit area of emitting surface, per unit solid angle, per unit wavelength, whereas 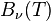 has units of energy emitted per unit time per unit area of emitting surface, per unit solid angle, per unit frequency. To be consistent, we must use the equality
has units of energy emitted per unit time per unit area of emitting surface, per unit solid angle, per unit frequency. To be consistent, we must use the equality
where both sides now have units of energy emitted per unit time per unit area of emitting surface, per unit solid angle.
Starting with the Rayleigh–Jeans law in terms of wavelength we get
where
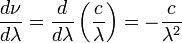 .
.
This leads us to find:
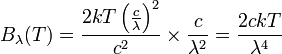 .
.
Other forms of Rayleigh–Jeans law
Depending on the application, the Planck Function can be expressed in 3 different forms. The first involves energy emitted per unit time per unit area of emitting surface, per unit solid angle, per unit frequency. In this form, the Planck Function and associated Rayleigh–Jeans limits are given by
or
Alternatively, Planck's law can be written as an expression  for emitted power integrated over all solid angles. In this form, the Planck Function and associated Rayleigh–Jeans limits are given by
for emitted power integrated over all solid angles. In this form, the Planck Function and associated Rayleigh–Jeans limits are given by
or
In other cases, Planck's Law is written as  for energy per unit volume (energy density). In this form, the Planck Function and associated Rayleigh–Jeans limits are given by
for energy per unit volume (energy density). In this form, the Planck Function and associated Rayleigh–Jeans limits are given by
or
See also
References
- ↑ Astronomy: A Physical Perspective, Mark L. Kutner pp. 15
- ↑ Radiative Processes in Astrophysics, Rybicki and Lightman pp. 20–28
- ↑ Astronomy: A Physical Perspective, Mark L. Kutner pp. 15