Ramipril
 | |
|---|---|
| Systematic (IUPAC) name | |
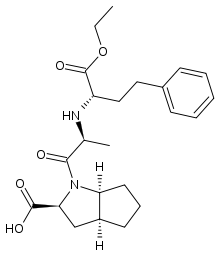
| (2S,3aS,6aS)-1-[(2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]-octahydrocyclopenta[b]pyrrole-2-carboxylic acid | |
| Clinical data | |
| Trade names | Altace |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a692027 |
| Pregnancy cat. | D |
| Legal status | POM (UK) ℞-only (US) |
| Routes | Oral |
| Pharmacokinetic data | |
| Bioavailability | 28% |
| Protein binding | 73% (ramipril) 56% (ramiprilat) |
| Metabolism | Hepatic, to ramiprilat |
| Half-life | 2 to 4 hours |
| Excretion | Renal (60%) and fecal (40%) |
| Identifiers | |
| CAS number | 87333-19-5 |
| ATC code | C09AA05 |
| PubChem | CID 5362129 |
| DrugBank | DB00178 |
| ChemSpider | 4514937 |
| UNII | L35JN3I7SJ |
| KEGG | D00421 |
| ChEBI | CHEBI:8774 |
| ChEMBL | CHEMBL1168 |
| Chemical data | |
| Formula | C23H32N2O5 |
| Mol. mass | 416.511 g/mol |
| SMILES
| |
| |
| | |
Ramipril is an angiotensin-converting enzyme (ACE) inhibitor, used to treat high blood pressure and congestive heart failure. It is marketed as Prilace by Arrow Pharmaceuticals in Australia, Ramipro by Westfield Pharma in the Philippines, Tritace by Sanofi-Aventis in Italy and United States and Altace by King Pharmaceuticals in the United States, Ramitens by PharmaSwiss, Ampril by Krka in Slovenia, Corpril by Cemelog-BRS in Hungary, Piramil and Prilinda by Hemofarm in Serbia, by Lek in Poland and by Novartis and Opsonin Pharma Limited as Ramace in Bangladesh, and in Canada as Altace (Sonfi) and Ramipril (Pharmascience).
Medical uses
Indications for its use include:
- Hypertension;
- Congestive heart failure;[1]
- Following heart attack in patients with clinical evidence of heart failure;
- Susceptible patients over 55 years: prevention of heart attack, stroke, cardiovascular death or need of revascularization procedures.
- Diabetic nephropathy (kidney damage due to diabetes) with microalbuminuria (protein in the urine)
Contraindications
Renovascular disease (impaired blood flow in the kidneys), severe renal impairment (especially in patients with one kidney or with bilateral renal artery stenosis), volume-depleted patients, history of angioedema while on an ACE inhibitor, pregnancy, hypotension.
Adverse effects
- low blood sugar (in patients taking medication for diabetes), causing sweating or shakiness
- dry cough
- dizziness and light-headedness due to low blood pressure
- tiredness and fatigue, especially in the early stages
- mouth dryness in the early stages
- nausea, vomiting, diarrhea (persistent in rare cases)
- fainting
- change in amount of urine
- signs of infection (e.g., fever, chills, persistent sore throat)
- yellowing of eyes or skin, dark urine
- stomach or abdominal pain
- neutropenia (low white blood cells)
- impotence (erectile dysfunction)[2]
Serious allergic reactions to this drug are unlikely, but immediate medical attention must be sought if they occur. Symptoms of a serious allergic reaction include, but are not limited to a rash or swelling of the face, mouth, tongue, or throat.
In extreme cases, ramipril may lead to potentially fatal liver problems.
Mechanism of action

Letter codes & icons may differ.
ACE inhibitors, as the name suggests, inhibit the actions of angiotensin converting enzyme (ACE), thereby lowering the production of angiotensin II and also decreasing the breakdown of bradykinin. The decrease in angiotensin II results in relaxation of arteriole smooth muscle leading to a decrease in total peripheral resistance, reducing blood pressure as the blood is pumped through widened vessels. Its effect on bradykinin is responsible for the dry cough side effect.
Ramipril, a prodrug or precursor drug, is converted to the active metabolite (metabolic product) ramiprilat by liver esterase enzymes.[3] Ramiprilat is mostly excreted by the kidneys. The half-life of ramiprilat is variable (3–16 hours), and is prolonged by heart and liver failure, as well as kidney failure.
Patent
The compound was protected by U.S. Patent 5,061,722 which was assigned to Aventis on 29 October 1991. The patent was scheduled to expire on 29 October 2008. On 11 September 2007, in an appeal by the Indian company Lupin Ltd., the United States Court of Appeals for the Federal Circuit reversed a district court trial verdict and found that Aventis's patent on ramipril was invalid for "obviousness" - opening this medicine to generic manufacturers.
Ramipril is marketed in India under the brand names of Cardace, Zigpril, Ramistar and Zorem.
Clinical trials
The HOPE trial[4][5] seemed to show ramipril possessed cardioprotective qualities which extended beyond its qualities as an antihypertensive. However, the HOPE trial and the interpretation of the results have been criticised.[6]
The AIRE trial[3][7] showed a 27% reduction in mortality.
Ramipril was found to have similar results as telmisartan, an angiotensin II receptor blocker (ARB).[8]
See also
References
- ↑ Pilote L, Abrahamowicz M, Eisenberg M, Humphries K, Behlouli H, Tu JV (May 2008). "Effect of different angiotensin-converting-enzyme inhibitors on mortality among elderly patients with congestive heart failure". CMAJ 178 (10): 1303–11. doi:10.1503/cmaj.060068. PMC 2335176. PMID 18458262.
- ↑ Med Tv
- ↑ 3.0 3.1 Frampton JE, Peters DH (March 1995). "Ramipril. An updated review of its therapeutic use in essential hypertension and heart failure". Drugs 49 (3): 440–66. PMID 7774515.
- ↑ Hypertension Online Slides - ramipril, mortality, kidney failure, HOPE
- ↑ Ramipril reduced mortality and cardiovascular morbidity in high risk adults - 5 (2): 47 - Evidence-Based Medicine
- ↑ http://www.medscape.com/viewarticle/430926 "Debate: Do ACE Inhibitors Have Unique Properties, Beyond Their Antihypertensive Effect?"
- ↑ "Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators". Lancet 342 (8875): 821–8. October 1993. doi:10.1016/0140-6736(93)92693-N. PMID 8104270.
- ↑ Yusuf S, Teo KK, Pogue J, et al (April 2008). "Telmisartan, ramipril, or both in patients at high risk for vascular events". N. Engl. J. Med. 358 (15): 1547–59. doi:10.1056/NEJMoa0801317. PMID 18378520.
External links
- Altace (ramipril), a registered trademark of King Pharmaceuticals, Inc.
- Data Sheet for Ramipril – ChemSpider 18 September 2008
- U.S. National Library of Medicine: Drug Information Portal - Ramipril
| ||||||||||||||||||||